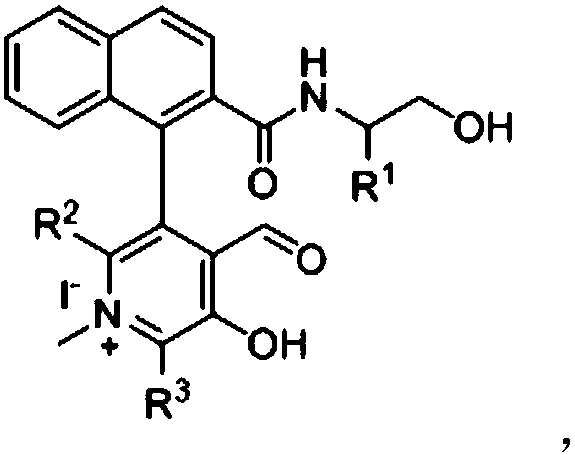
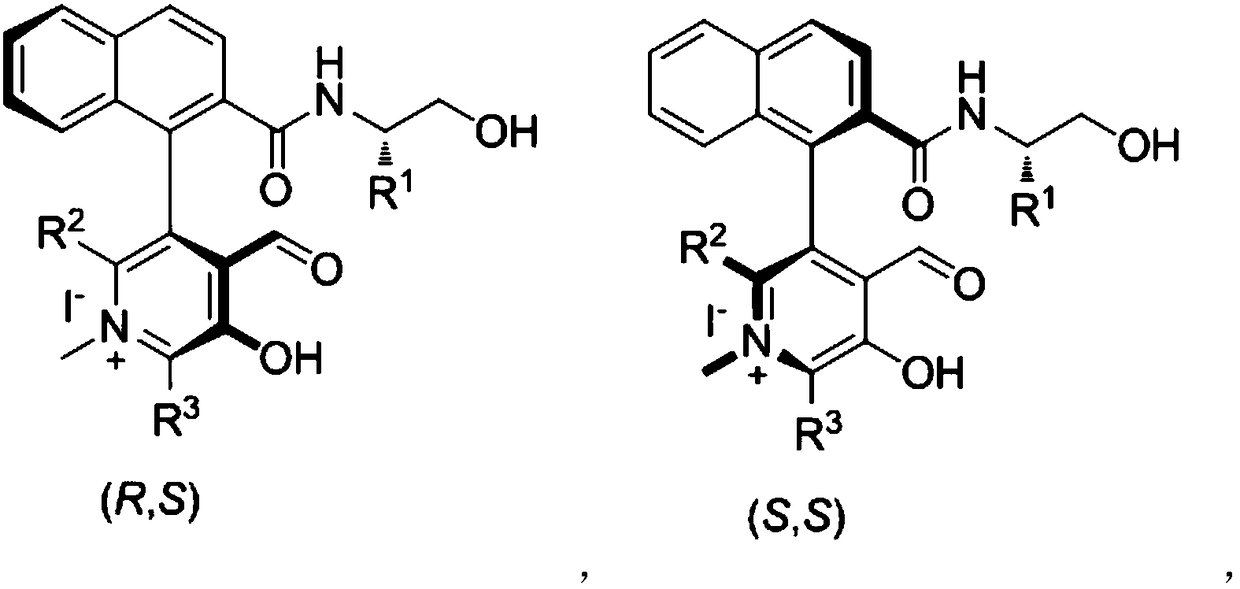
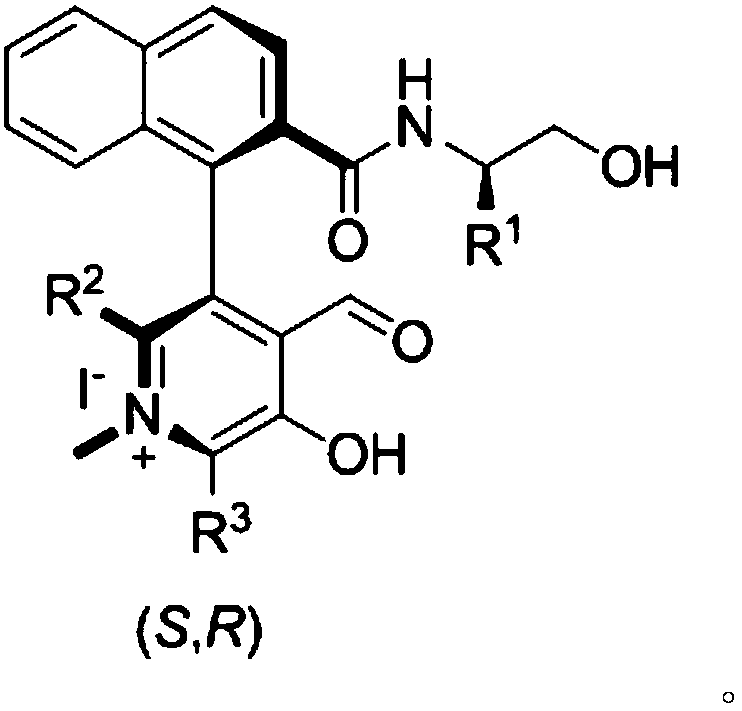
Novel biaryl-structured chiral N-methyl pyridoxal catalyst and synthesis and application thereof
A methyl pyridoxal and catalyst technology, applied in catalytic reaction, physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of α-β-diamino acid derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The synthesis of embodiment 1 compound 7:
[0064]
[0065] Add compound 5 (2.00g, 7.69mmol), compound 6 (2.31g, 11.54mmol), palladium complex (0.158g, 0.19mmol), ligand Ruphos (0.358g, 0.77mmol) and KF (1.34g, 23.07mmol), add a stirring bar, cover the rubber stopper, replace N 2 Three times, then inject 1,4-dioxane (20 mL) and water (2.5 mL) by syringe. After the reaction system was stirred at room temperature for 30 minutes, it was transferred to 100° C. and stirred overnight. Cool to room temperature, filter, concentrate the filtrate, and obtain compound 7 (yellow oily liquid, 0.93 g, yield 36%) by column chromatography.
[0066] Yellow oil; 1 H NMR (400MHz, CDCl 3 )δ10.19(s,1H),9.82(s,1H),8.82(s,1H),8.07(d,J=8.8Hz,1H),8.01(d,J=8.8Hz,1H),7.96( d, J=8.4Hz, 1H), 7.63(t, J=7.6Hz, 1H), 7.44(t, J=8.0Hz, 1H), 7.27(d, J=7.2Hz, 1H), 5.41(s, 2H), 3.60(s,3H), 2.07(s,3H).
Embodiment 1-1
[0068] Compared with Example 1, except that the molar ratio of compound 5 and compound 6 was replaced by 1:1, and the reaction conditions were replaced by: reaction temperature -20°C, reaction time 24h, the rest were the same.
Embodiment 1-2
[0070] Compared with Example 1, except that the molar ratio of Compound 5 and Compound 6 was replaced by 1:5, and the reaction conditions were replaced by: reaction temperature 120° C., reaction time 1 h, the rest were the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com