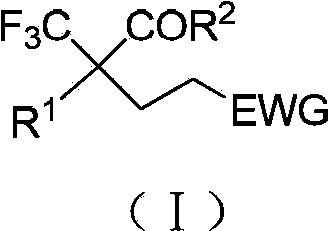
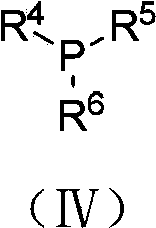Preparation method of trifluoromethyl carbonyl compound
A technology of compound and synthesis method, which is applied in the field of trifluoromethyl-containing organic compounds, and can solve the problems of low environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In a 10ml reaction tube, add 2-(trifluoromethyl)-3,3,3-trifluoropropionic acid methyl ester (0.2mmol), then add 2-dicyclohexylphosphine-2,4,6-triiso Propyl biphenyl (X-PHOS) (0.02mmol), then add 1mL N,N-dimethylformamide, and finally add phenyl vinyl ketone (0.6mmol), stir the reaction at 30°C for 1h, the reaction is complete, The conversion rate of 2-(trifluoromethyl)-3,3,3-trifluoropropionic acid methyl ester is 100% and the yield of fluorine spectrum is 99%. The product is obtained by column chromatography with an isolated yield of 95%. , the product structure and analysis data are as follows:
[0036] 5-Phenyl-2,2-bis(trifluoromethyl)-5-oxopentanoic acid methyl ester, 1 H NMR (300MHz, CDCl 3 )δ7.95(d,J=7.8Hz,2H),7.59(t,J=7.2Hz,1H),7.48(t,J=7.2Hz,2H),3.91(s,3H),3.14(t, J=7.8Hz,2H),2.65(t,J=7.8Hz,2H); 19 F NMR (282MHz, CDCl 3 )δ-66.2; HRMS (ESI) calcd for C 14 h 12 f 6 o 3 Na[M+Na] + 365.0581found 365.0582.
Embodiment 2~12
[0038] Similar to Example 1, X-PHOS was selected as the catalyst, methyl 2-(trifluoromethyl)-3,3,3-trifluoropropionate (0.2 mmol) was used as the reaction raw material, the solvent DMF1mL was added, and different substituted Phenyl vinyl ketones were reacted using the same analytical means as in Example 1. The reaction conditions and results are shown in Table 1.
[0039] Table 1
[0040]
[0041] Table 1
[0042]
Embodiment 2
[0043] The product structure and analysis data of embodiment 2 are as follows:
[0044] Methyl 5-(4-chlorophenyl)-2,2-bis(trifluoromethyl)-5-oxopentanoate, 1 H NMR (300MHz, CDCl 3 )δ7.89(d,J=8.4Hz,2H),7.46(d,J=8.4Hz,2H),3.92(s,3H),3.11(t,J=7.8Hz,2H),2.63(t, J=7.8Hz,2H); 19 F NMR (282MHz, CDCl 3 )δ-66.2; HRMS (EI) calcd for C 14 h 11 CIF 6 o 3 [M] + 376.0306 found 376.0301.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com