Synthesis method of photocatalytic tertiary amine compound
A technology of tertiary amine compounds and synthetic methods, applied in the preparation of organic compounds, preparation of amino compounds from amines, chemical instruments and methods, etc., can solve the problems of weak redox ability, limited application, high price, etc. Strong universality, easy operation and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
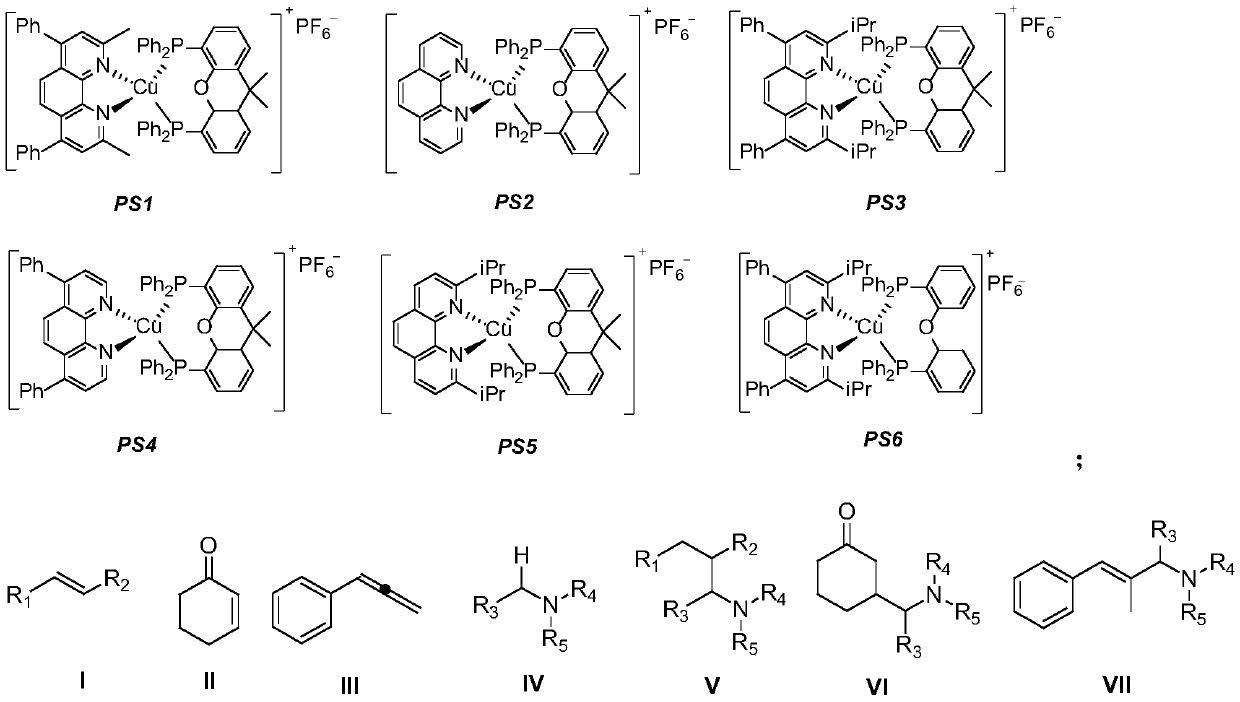
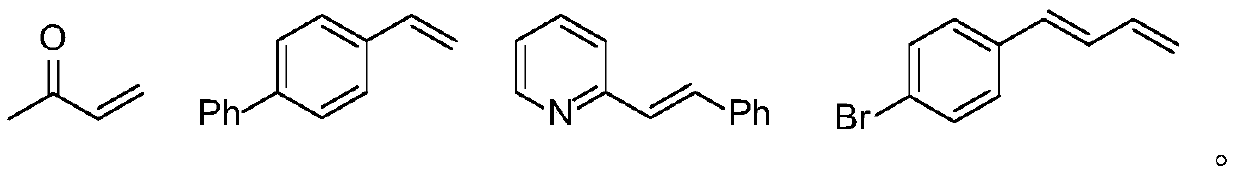
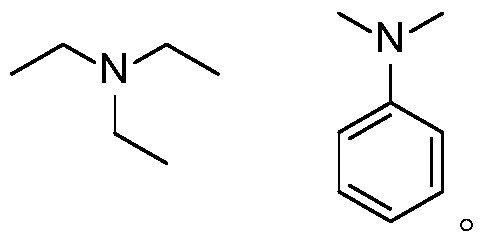
[0037] Photosensitizer PS1 (11.5mg, 0.01mmol) and potassium acetate (39.3mg, 0.4mmol) were added to the Schlenk reaction tube, vacuumed and replaced with nitrogen three times, and then the formula 4-phenylstyrene (36mg, 0.2mmol) and three Ethylamine (40.5mg, 0.4mmol) was dissolved in tetrahydrofuran (2mL), added to the above reaction tube under the protection of nitrogen, and under the irradiation of 15W blue LED, stirred and reacted at 25°C for 12 hours. After the reaction was completed, the obtained 100-200 mesh column chromatography silica gel was added to the reaction solution and the solvent was distilled off under reduced pressure, and the obtained crude product was separated by silica gel column chromatography, and eluted with petroleum ether / ethyl acetate=8 / 1 as the eluent, TLC followed the elution process, collected the eluent containing the target product, combined the eluent and evaporated the solvent to obtain the pure product (31.5 mg). The material wa...
Embodiment 2
[0040]
[0041] Photosensitizer PS3 (12.0mg, 0.01mmol) and potassium acetate (39.3mg, 0.4mmol) were added into the Schlenk reaction tube, vacuumed and replaced with nitrogen three times, and then the formula 4-phenylstyrene (36mg, 0.2mmol) and three Ethylamine (40.5mg, 0.4mmol) was dissolved in tetrahydrofuran (2mL), added to the above reaction tube under the protection of nitrogen, and under the irradiation of 15W blue LED, stirred and reacted at 25°C for 12 hours. After the reaction was completed, the obtained 100-200 mesh column chromatography silica gel was added to the reaction solution and the solvent was distilled off under reduced pressure, and the obtained crude product was separated by silica gel column chromatography, and eluted with petroleum ether / ethyl acetate=8 / 1 as the eluent, TLC followed the elution process, collected the eluent containing the target product, combined the eluent and evaporated the solvent to obtain the pure product. The material was a colo...
Embodiment 3
[0044]
[0045] Photosensitizer PS5 (10.5mg, 0.01mmol) and potassium acetate (39.3mg, 0.4mmol) were added to the Schlenk reaction tube, vacuumed and changed for nitrogen three times, and then the formula 4-phenylstyrene (36mg, 0.2mmol) and three Ethylamine (40.5mg, 0.4mmol) was dissolved in tetrahydrofuran (2mL), added to the above reaction tube under the protection of nitrogen, and under the irradiation of 15W blue LED, stirred and reacted at 25°C for 12 hours. After the reaction was completed, the obtained 100-200 mesh column chromatography silica gel was added to the reaction solution and the solvent was distilled off under reduced pressure, and the obtained crude product was separated by silica gel column chromatography, and eluted with petroleum ether / ethyl acetate=8 / 1 as the eluent, TLC followed the elution process, collected the eluent containing the target product, combined the eluent and evaporated the solvent to obtain the pure product. The material was a colorles...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com