A method for one-step synthesis of carboxylic acids with two extended carbon chains from olefins
A technology of olefins and carbon chains, which is applied in the field of carboxylic acid compound synthesis, can solve the problems of unsuitable application, cumbersome steps, harsh reaction conditions, etc., and achieve the goal of improving atom and step economy, avoiding mutual conversion, and mild synthesis conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
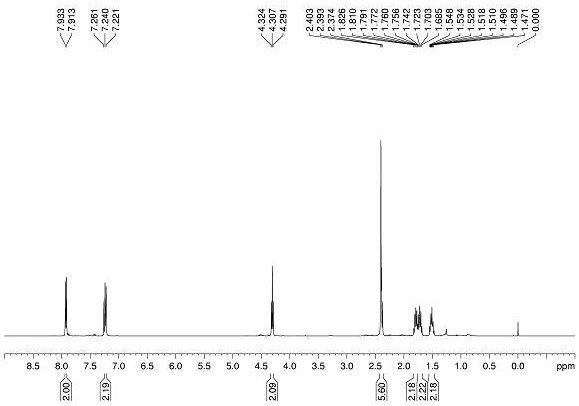
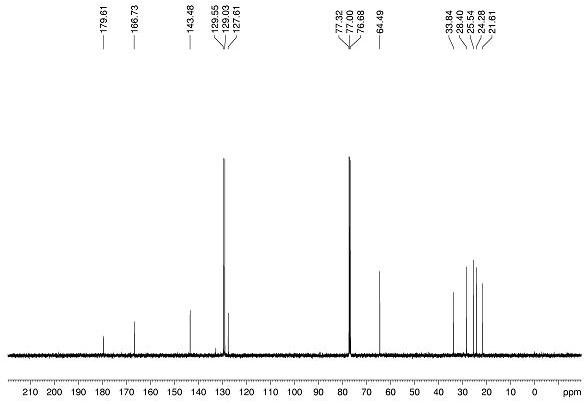
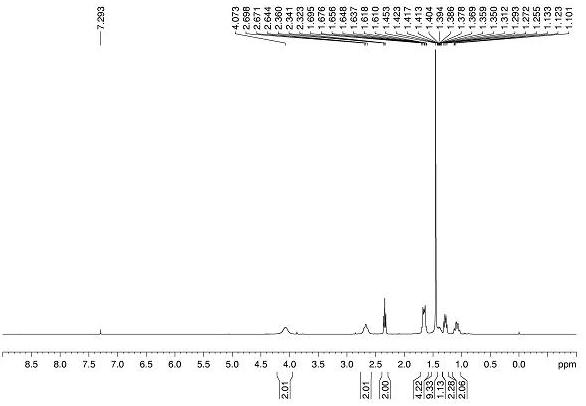
Image
Examples
Embodiment 1
[0074]
[0075] Alkene substrate 1a (0.2 mmol), bromoacetic acid 2a (0.4 mmol), Hans ester HE (0.4 mmol), diphenyl disulfide (0.06 mmol, 30 mol%) and photocatalyst [Ir(ppy) 2 dtbbpy]PF 6 (0.002 mmol, 1 mol%) was added to a dry glass reaction tube with magnetons, 1.5 mL of dry 1,4-dioxane was added as a solvent, and the reaction tube was sealed, degassed by freezing three times, and then injected into Trifluoroacetic acid (0.3 mmol), and then continue to seal the reaction system under nitrogen protection. The reaction was transferred to 25W blue light irradiation, and the reaction was carried out at room temperature for 12 hours. After the reaction was complete, transfer the reaction system to a 25 mL round-bottomed flask, add 8 mL of ethyl acetate to dilute, then alkalize with 10 mL of saturated sodium bicarbonate solution, stir for half an hour and extract 3 times with ethyl acetate, Collect the aqueous phase in an Erlenmeyer flask; combine the three extracted organic ph...
Embodiment 2
[0078]
[0079] Alkene substrate 1a (0.2 mmol), bromoacetic acid 2a (0.4 mmol), Hans ester HE (0.4 mmol), diphenyl disulfide (0.06 mmol, 30 mol%) and photocatalyst fac -Ir(ppy) 3 (0.002 mmol, 1 mol%) was added to a dry glass reaction tube with magnetons, 1.5 mL of dry 1,4-dioxane was added as a solvent, and the reaction tube was sealed, degassed by freezing three times, and then injected into Trifluoroacetic acid (0.3 mmol), and then continue to seal the reaction system under nitrogen protection. The reaction was transferred to 25W blue light irradiation, and the reaction was carried out at room temperature for 12 hours. After the reaction was complete, transfer the reaction system to a 25 mL round-bottomed flask, add 8 mL of ethyl acetate to dilute, then alkalize with 10 mL of saturated sodium bicarbonate solution, stir for half an hour and extract 3 times with ethyl acetate, Collect the aqueous phase in an Erlenmeyer flask; combine the three extracted organic phases an...
Embodiment 3
[0081]
[0082] Alkene substrate 1a (0.2 mmol), bromoacetic acid 2a (0.4 mmol), Hans ester HE (0.4 mmol), diphenyl disulfide (0.06 mmol, 30 mol%) and photocatalyst [Ir(dF(CF 3 )ppy 2 )dtbbpy]PF 6 (0.002 mmol, 1 mol%) was added to a dry glass reaction tube with magnetons, 1.5 mL of dry 1,4-dioxane was added as a solvent, and the reaction tube was sealed, degassed by freezing three times, and then injected into Trifluoroacetic acid (0.3 mmol), and then continue to seal the reaction system under nitrogen protection. The reaction was transferred to 25W blue light irradiation, and the reaction was carried out at room temperature for 12 hours. After the reaction was complete, transfer the reaction system to a 25 mL round-bottomed flask, add 8 mL of ethyl acetate to dilute, then alkalize with 10 mL of saturated sodium bicarbonate solution, stir for half an hour and extract 3 times with ethyl acetate, Collect the aqueous phase in an Erlenmeyer flask; combine the three extracted ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com