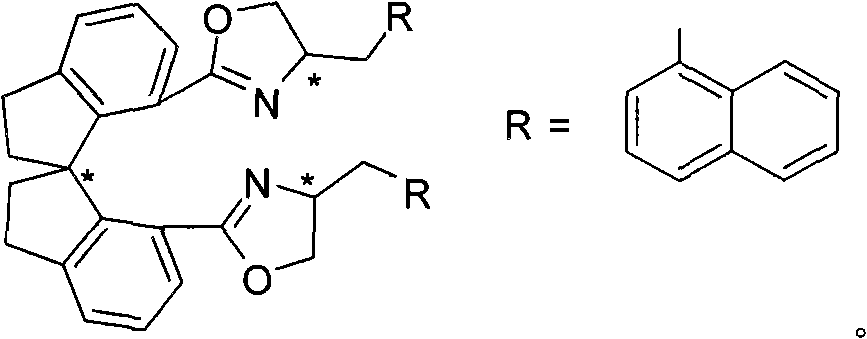
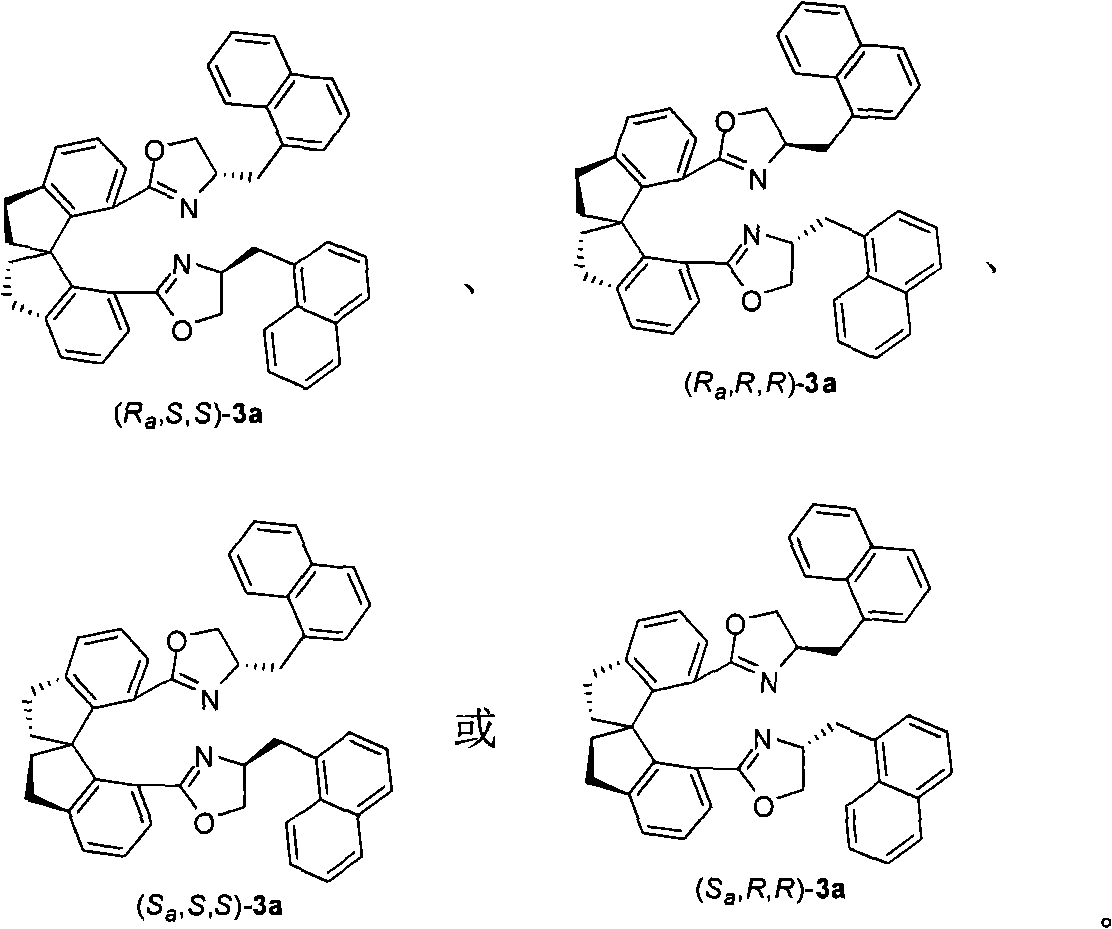
Alpha-menaphthyl substituted spiro bis(oxazoline) ligands, synthetic method and application thereof in synthesizing pyrazolidine derivatives
A technology of bisoxazoline and spiro ring, which is applied in the field of synthesis of spiro bisoxazoline ligands, can solve limited problems, achieve strong regulation ability, high catalytic activity and chiral induction effect, and simple synthesis method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
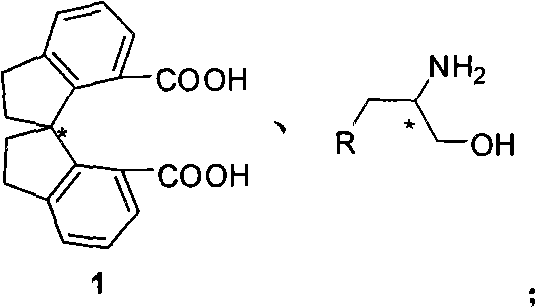
[0032] Under inert gas protection, add 308mg (R a )-1, 442mg aminoalcohol, 869mg DCC and 297mg nitrogen hydroxybenzotriazole. Add 40 mL of tetrahydrofuran under ice-cooling, keep at 0°C for 1-10 hours, then naturally rise to room temperature, react overnight, and TLC detects that the reaction is complete (petroleum ether: ethyl acetate = 1:1). The solvent was removed under reduced pressure, and the product was directly used in the next reaction by short column filtration.
[0033] Add 13 mg of DMAP to the reaction tube containing the product obtained in the previous step. After ventilating, add 30 mL of dichloromethane, add 0.68 mL of triethylamine under ice bath, add 0.34 mL of methanesulfonyl chloride after stirring for a while, and keep the reaction at 0°C After 0.5-5 hours, add 2.9 mL of triethylamine, let it rise to room temperature naturally, and react overnight, and TLC detects that the reaction is complete. Add 100mL ether to the system for extraction, th...
Embodiment 2
[0036] (R 1 =Bn,R 2 =Bn,R 3 =Ph)
[0037] Pd(dba) 2 (3mg, 0.0053mmol) and (R a , S, S)-3 (4mg, 0.0063mmol) (ligand synthesized in Example 1) was complexed in 1mL tetrahydrofuran for 0.5-3 hours, then added Ag 3 PO 4 (19mg, 0.045mmol), 4a (64mg, 0.10mmol), iodobenzene 5a (25mg, 0.12mmol) and 1mL tetrahydrofuran. The reaction was reacted at 0-200°C, TLC followed the end of the reaction, and directly spin-dried column chromatography yielded no Viscous liquid 61 mg (R)-6aa (85% yield, 93% ee). 1 H NMR (400MHz, CDCl 3 ): δ7.28-7.06(m, 21H), 7.05-6.98(m, 4H), 5.55(s, 1H), 5.44-5.38(m, 1H), 5.13-4.78(m, 9H), 3.00(dd , J=13.2, 8.4Hz, 1H), 2.47(dd, J=13.2, 2.8Hz, 1H); 13 C NMR (100.5MHz, CDCl 3 ): δ167.8, 166.0, 157.0, 153.3, 143.6, 138.4, 135.5, 135.3, 134.9, 134.4, 128.40, 128.36, 128.2, 128.15, 128.12, 128.07, 128.0, 127.8, 71.5, 127.6, 123.6 68.3, 68.2, 68.0, 61.3, 41.3; MS (ESI): m / z 749 (M+K + ), 733 (M+Na + ); IR (neat): 1738, 1586, 1498, 1455, 1397, 1337, 1276, 118...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com