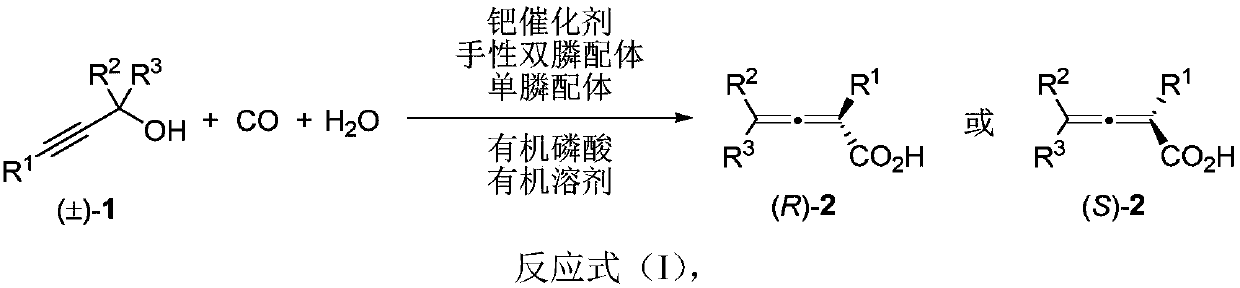
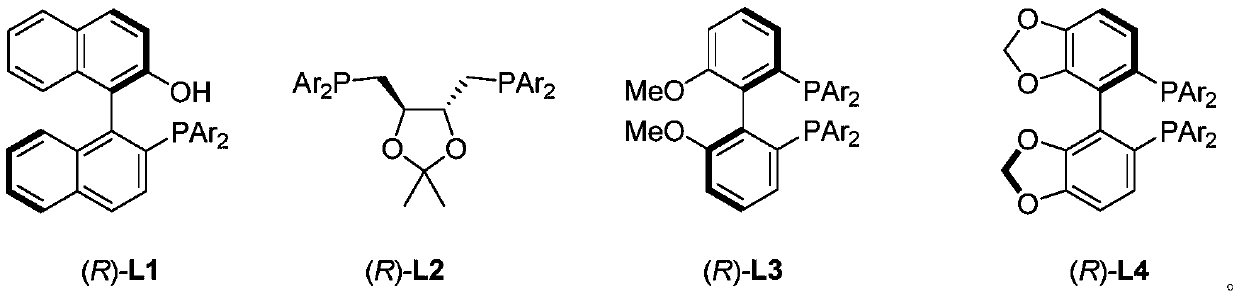
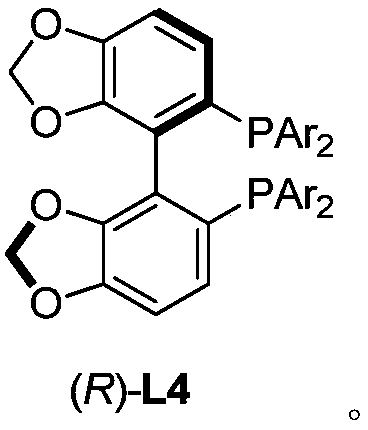
Method for directly constructing tetra-substituted allenic acid compound with high optical activity
An optically active, allenoic acid technology, applied in the preparation of organic compounds, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of narrow substrate range, poor atomic economics, and low reaction yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]
[0048] Wherein, mol means mole, toluene means toluene, COballoon means carbon monoxide balloon, ee means enantiomeric excess percentage.
[0049] Add PdCl sequentially to a dry Schlenk reaction tube 2 (0.0036g, 0.02mmol), chiral bisphosphine ligand (R)-L4c (0.057g, 0.048mmol), monophosphine ligand PPh 3 (0.0527g, 0.2mmol), and (PhO) 2 PO 2H (0.0501 g, 0.2 mmol). After the reaction tube was plugged with a rubber stopper, the vacuum pump was connected, and the argon gas was replaced three times under an argon atmosphere. ), water (360 μL, d=1.0 g / mL, 0.36 g, 20 mmol), toluene (2 mL). After turning off the argon gas, place the reaction tube in a liquid nitrogen bath to freeze for 3 minutes, insert a carbon monoxide balloon (about 1 liter), and replace the carbon monoxide three times in a carbon monoxide atmosphere, then remove the liquid nitrogen bath, and wait for the reaction system to return to room temperature and melt into a liquid , place the reaction tube ...
Embodiment 2
[0051]
[0052] Operation is with embodiment 1. PdCl 2 (0.0036g, 0.02mmol), (R)-L4c (0.0564g, 0.048mmol), PPh 3 (0.0523g, 0.2mmol), (PhO) 2 PO 2 H (0.05g, 0.2mmol), (±)-1b (0.2157g, 1mmol), water (360μL, d=1.0g / mL, 0.36g, 20mmol), toluene (5mL), at 0°C, reaction 18 Hour. Flash column chromatography (eluent: petroleum ether (60-90°C) / diethyl ether / dichloromethane=30 / 1 / 1, petroleum ether (60-90°C) / ethyl acetate=8 / 1) obtained chiral Allenoic acid product (S)-2b (0.1029g, 42%): oil; 96%ee (HPLC conditions: AS-H column, hexane / i-PrOH=98 / 2, 1.0mL / min, λ=214nm ,t R (major)=6.5min,t R (minor)=9.3min); [α] D 26 =+106.7 (c=1.50, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 ):δ=7.29-7.22(m,1H,Ar-H),7.22-7.12(m,3H,Ar-H),2.40(s,3H,CH 3 ),2.35-2.05(m,5H,CH 2 and CH 3 ),1.55-1.40(m,2H,CH 2 ),1.40-1.27(m,2H,CH 2 ),0.90(t,J=7.2Hz,3H,CH 3 ); 13 C NMR (100MHz, CDCl 3 ):δ=210.2,173.8,136.2,136.0,130.6,127.9,127.6,125.9,104.5,98.9,30.1,28.1,22.2,20.4,19.9,13.8; IR(neat):v=3200-2410(br)...
Embodiment 3
[0054]
[0055] Operation is with embodiment 1. PdCl 2 (0.0036g, 0.02mmol), (R)-L4c (0.0571g, 0.048mmol), PPh 3 (0.0526g, 0.2mmol), (PhO) 2 PO 2 H (0.0499g, 0.2mmol), (±)-1c (0.2165g, 1mmol), water (360μL, d=1.0g / mL, 0.36g, 20mmol), toluene (5mL), at -5 ℃, the reaction 18 hours. Flash column chromatography (eluent: petroleum ether (60-90°C) / diethyl ether / dichloromethane=30 / 1 / 1, petroleum ether (60-90°C) / ethyl acetate=8 / 1) obtained chiral Allenoic acid product (S)-2c (0.0687g, 28%): solid; 94%ee (HPLC conditions: AS-H column, hexane / i-PrOH=98 / 2, 1.0mL / min, λ=214nm, t R (major)=6.5min,t R (minor)=7.8min); [α] D 26 =+16.5 (c=1.00, CHCl 3 ). Melting point: 96.8-98.5°C (measured directly after the solvent evaporates to dryness). 1 H NMR (400MHz, CDCl 3 ):δ=7.27-7.20(m,1H,Ar-H),7.20-7.15(m,2H,Ar-H),7.07(d,J=7.2Hz,1H,Ar-H),2.40-2.28( m,5H,CH 2 and CH 3 ),2.18(s,3H,CH 3 ),1.51-1.41(m,2H,CH 2 ),1.41-1.28(m,2H,CH 2 ), 0.88(t, J=7.4Hz, 3H, CH 3 ); 13 C NMR (100MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com