Chirality phosphine nitrogen compound containing N-aryl as well as synthetic method and application thereof
A synthesis method and compound technology, which can be applied to organic compounds/hydrides/coordination complex catalysts, compounds of Group 5/15 elements of the periodic table, chemical instruments and methods, etc., which can solve the problem of expensive, diastereomeric selection It can solve the problems of poor control of properties and poor control of enantioselectivity, so as to achieve the effect of expanding the scope of use, high enantioselectivity and excellent yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
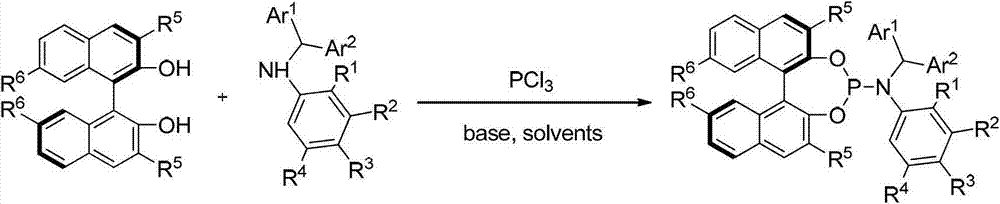
[0043] Embodiment 1: Synthesis based on axial chiral N-arylphosphine nitrogen compounds:
[0044]
[0045] Under argon protection, add toluene (50mL) and triphenylphosphine (0.67mL, 7.7mmol) into a dry 250ml three-necked flask, cool to 0°C; in another dry 25ml flask In , add aromatic secondary amine (7.7mmol), toluene (8mL), and triethylamine (1.8mL, 12.9mmol) to make a mixed solution, and then gradually drop the mixed solution into the above 250ml flask. After the dropwise addition, the temperature was raised to 80°C for 6 hours, and then gradually cooled to 0°C. A solution of binaphthyl diol (BINOL) (7.0 mmol) and triethylamine (3.5 mL, 25.2 mmol) in toluene (30 mL) and tetrahydrofuran (6 mL) was slowly added to the system. The system was stirred overnight at room temperature (25°C), filtered through celite, and the solvent was distilled off under reduced pressure, and the crude product was separated by column chromatography (petroleum ether / ethyl acetate / triethylamine: ...
Embodiment 2
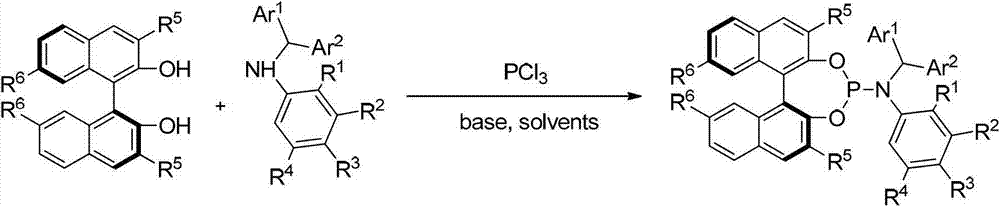
[0154] Embodiment 2: Synthesis based on axial chiral N-arylphosphine nitrogen compounds:
[0155]
[0156] Under argon protection, add toluene (50mL) and triphenylphosphine (0.67mL, 7.7mmol) into a dry 250ml three-necked flask, cool to 0°C; in another dry 25ml flask In , add aromatic secondary amine (7.7mmol), toluene (8mL), and triethylamine (1.8mL, 12.9mmol) to make a mixed solution, and then gradually drop the mixed solution into the above 250ml flask. After the dropwise addition, the temperature was raised to 80°C for 6 hours, and then gradually cooled to 0°C. A solution of diphenol 2 (7.0 mmol) and triethylamine (3.5 mL, 25.2 mmol) in toluene (30 mL) and tetrahydrofuran (6 mL) was slowly added to the system. The system was stirred overnight at room temperature (25°C), filtered through celite, and the solvent was distilled off under reduced pressure, and the crude product was separated by column chromatography (petroleum ether / ethyl acetate / triethylamine: 20 / 1 / 0.01). ...
Embodiment 3
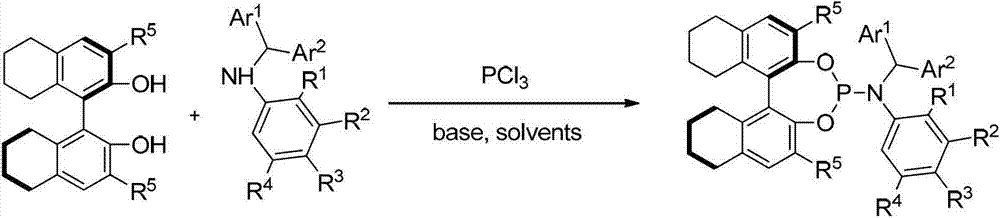
[0171] Embodiment 3: Synthesis based on axial chiral N-arylphosphine nitrogen compounds:
[0172]
[0173] Under argon protection, in a dry 250 ml three-necked flask, add toluene (50 mL) and triphenylphosphine (0.67 mL, 7.7 mmol), cool to 0 °C; in another dry 25 ml flask , Add aromatic secondary amine (7.7mmol), toluene (8mL), and triethylamine (1.8mL, 12.9mmol) to prepare a mixed solution, and then gradually drop the mixed solution into the above-mentioned 250ml flask. After the dropwise addition was completed, the temperature was raised to 80° C. for 6 hours, and then gradually cooled to 0° C. A solution of diphenol 3 (7.0 mmol) and triethylamine (3.5 mL, 25.2 mmol) in toluene (30 mL) and tetrahydrofuran (6 mL) was slowly added to the system. The system was stirred overnight at room temperature (25°C), filtered through celite, and the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (petroleum ether / ethyl acetate / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com