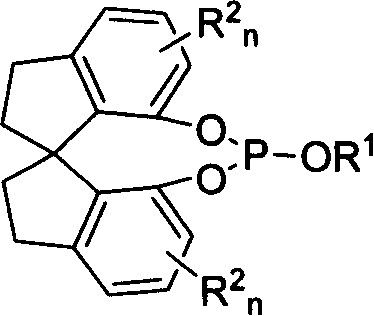
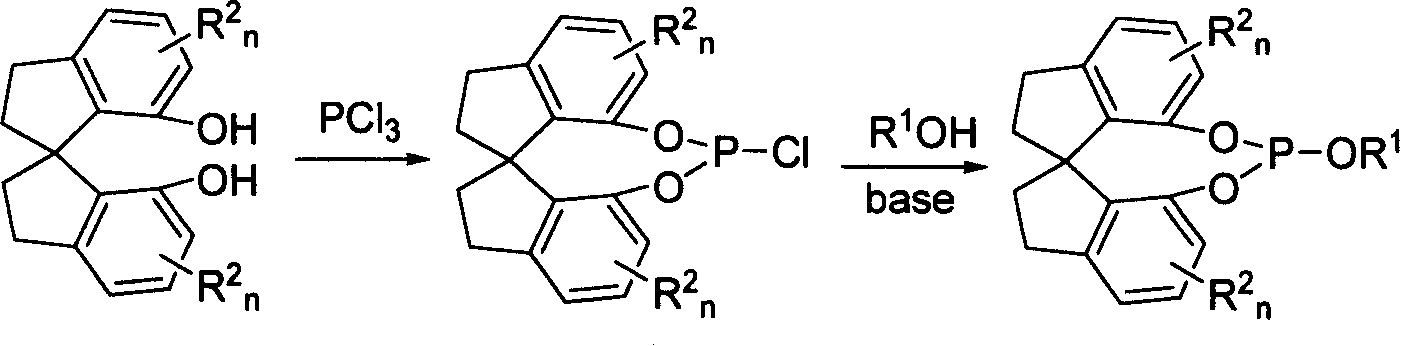
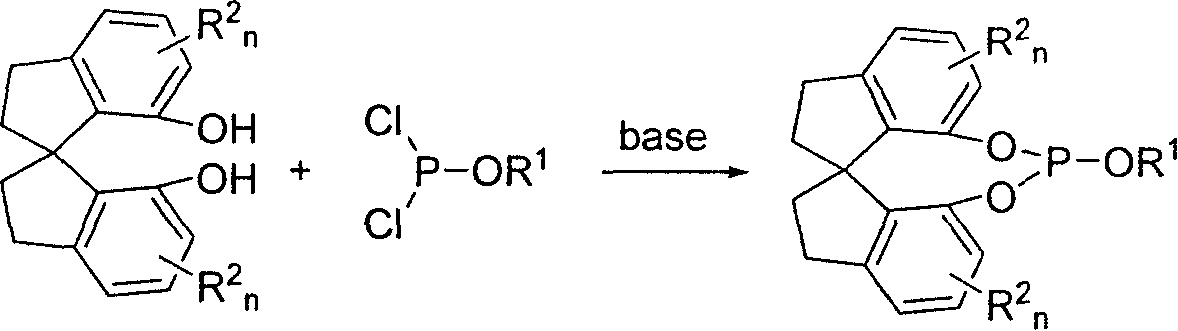New type spirocyclic phosphic ester, preparation method and application in asymmetric addition reaction
A technology of spirocyclic phosphite and aryl phosphite, which is applied in the field of novel chiral spirocyclic monophosphorous ligands, can solve the problems of low enantioselectivity and inability to obtain ideal results, and achieve a simple and efficient synthesis method. The effect of convenient group construction and high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of (R)-O, O'-[7,7'-(1,1'-spirodihydroindene)]-O-methyl phosphite
[0039]
[0040] Under a nitrogen atmosphere, add 263 mg (2.6 mmol) triethylamine to a reaction flask containing 308 mg (1.2 mmol) (R)-spirocyclic diphenol and 15 mL of dichloromethane, and add 263 mg (2.6 mmol) of triethylamine to the reaction flask at 0°C under stirring. 174 mg (1.3 mmol) of methoxyphosphorus dichloride was added dropwise, and the reaction was maintained at 0° C. for 0.5 hours, and then reacted at room temperature for 3 hours. The obtained reaction solution was desolventized under reduced pressure, and the residue was dissolved in n-hexane, filtered through diatomaceous earth, and 318 mg of white solid was obtained after desolventization. Yield: 85%. Melting point: 135-137°C. [α] D 20 =-522.8 (c 0.5, CH 2 Cl 2 ); 1 H NMR: δ7.21-7.14 (m, 2H, Ar-H), 7.06 (t, J=8Hz, 2H, Ar-H), 6.92 (d, J=7.8Hz, 1H, Ar-H), 6.78 (d, J=7.8Hz, 1H, Ar-H), 3.46 (d, J=10.1Hz, 3H,...
Embodiment 2
[0041] Example 2: Preparation of (R)-O, O'-[7,7'-(1,1'-spirodihydroindane)-O-phenyl phosphite
[0042]
[0043] Under a nitrogen atmosphere, add 263 mg (2.6 mmol) of triethylamine to a reaction flask containing 308 mg (1.2 mmol) (R)-spirocyclic diphenol and 15 mL of toluene, and dropwise add Phosphorus trichloride 178mg (1.3mmol), then react at room temperature for 20 hours, filter through diatomaceous earth, add 113mg (1.2mmol) of phenol to the obtained filtrate, add triethylamine 152mg (1.5mmol) under stirring, and react at room temperature After 3 hours, the solvent was removed, and the residue was subjected to silica gel column chromatography (eluent: ethyl acetate / petroleum ether=1:10) to obtain 336 mg of a white solid. Yield: 75%. The melting point is 104-105°C. [α] D 25 =+72(c 0.5, CHCl 3 ). 1 H NMR: δ7.35-6.77(m, 11H), 3.12-3.03(m, 2H), 2.87-2.80(m, 2H), 2.28-2.22(m, 2H), 2.07-2.00(m, 2H). 13 C NMR: δ151.2, 145.1, 144.7, 143.8, 142.3, 141.9, 138.7, 128.7, 127...
Embodiment 3
[0044] Example 3: Preparation of (R)-O, O'-[7,7'-(1,1'-spirodihydroindene)]-O-tert-butyl phosphite
[0045] The phenol in Example 2 was replaced with tert-butanol, and the preparation method was the same as in Example 2 to obtain (R)-O, O'-[7,7'-(1,1'-spirodihydroindene)]-O - tert-butyl phosphite, white solid, yield: 65%. Melting point: 57-58°C. [α] D 20 =+80(c0.5, CHCl 3 ). 1 H NMR: δ7.20-6.76(m, 6H), 3.08-3.02(m, 2H), 2.85-2.81(m, 2H), 2.24-2.18(m, 2H), 2.04-2.00(m, 2H), 1.48(s, 9H). 13 C NMR: δ145.1, 14.8, 144.4, 142.9, 142.0, 139.1, 127.3, 126.2, 121.8, 120.8, 120.5, 119.9, 58.0, 37.4, 36.9, 29.9 9.5. 31 P NMR: δ128.1.HR-MS (FAB) (theoretical) C 21 h 23 o 3 P+H: 355.1463 (355.1457).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com