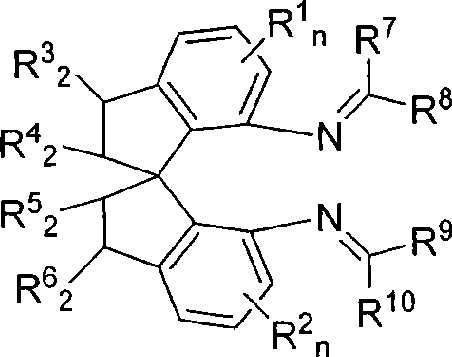
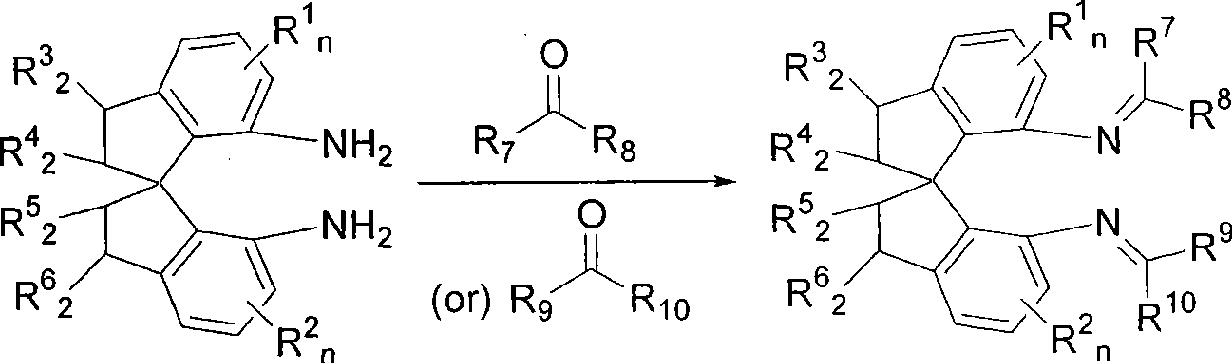
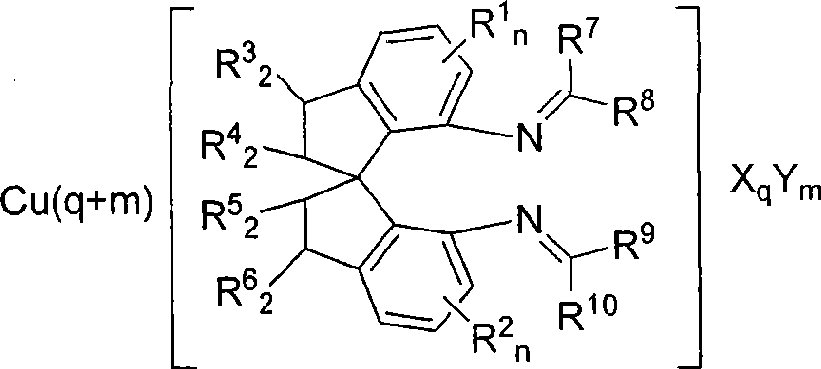
Spiro bisimine and preparation method and application thereof
A bis-imine, bis-imine copper technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problems of lack of chiral catalysts and no ideal results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: (R)-N, N'-[1-(2,6-dichlorophenyl)-methylene]-1,1'-spirodihydroindane-7,7'-diamine
[0024]
[0025] Add (R)-1,1'-spirodihydroindane-7,7'-diamine (800mg, 3.2 mmol), 2,6-dichlorobenzaldehyde (1.56g, 9.0mmol) and 9.6g Molecular sieve, replace the system with nitrogen atmosphere. Add freshly distilled toluene (60mL) and freshly distilled NEt to the system 3 (1.9 mL), stirred and reacted at 80°C for three days, filtered and washed the filter residue with dichloromethane (20 mL), combined the filtrates, and recrystallized the residue obtained after rotary evaporation and precipitation with ethanol to obtain a yellow solid with a yield of 60%. Melting point: 128-130°C. -21.0 (c0.5, CH 2 Cl 2 ). 1 H NMR (300MHz, CDCl 3 ): δ8.37(s, 2H), 7.14-6.90(m, 10H), 6.50-6.48(m, 2H), 3.03-2.89(m, 4H), 2.53-2.43(m, 2H), 2.20-2.14 (m, 2H). 13 C NMR (75MHz, CDCl 3 ): δ154.7, 148.6, 144.9, 142.1, 135.6, 131.5, 130.1, 128.8, 127.5, 122.2, 116.0, 60.5, 38.2, 31.2.
Embodiment 2
[0026] Example 2: (R)-N, N'-[1-(2,4-dichlorophenyl)-methylene]-1,1'-spirodihydroindane-7,7'-diamine
[0027]
[0028] The synthesis method is the same as in Example 1, with 2,4-dichlorobenzaldehyde replacing 2,6-dichlorobenzaldehyde. The product is a yellow solid with a yield of 56%. Melting point: 181-183°C. +287(c0.5, CH 2 Cl 2 ). 1 H NMR (300MHz, CDCl 3 ): δ8.38(s, 2H), 7.28-7.25(m, 2H), 7.14-7.13(m, 4H), 6.99-6.95(m, 2H), 6.77-6.66(m, 4H), 3.16-2.96 (m, 4H), 2.43-2.28 (m, 4H). 13 C NMR (75MHz, CDCl 3 ): δ152.9, 147.2, 144.3, 143.6, 136.8, 135.6, 132.2, 129.7, 129.1, 127.5, 127.2, 122.5, 115.1, 60.1, 38.0, 31.2.
Embodiment 3
[0029] Example 3: (R)-N, N'-[1-(2-chlorophenyl)-methylidene]-1,1'-spirodihydroindane-7,7'-diamine
[0030]
[0031] The synthetic method is the same as in Example 1, and 2,6-dichlorobenzaldehyde is replaced with 2-chlorobenzaldehyde. The product is a yellow solid with a yield of 94%. Melting point: 138-140°C. +181(c0.5, CH 2 Cl 2 ). 1 H NMR (300MHz, CDCl 3 ): δ 8.64(s, 2H), 7.38-7.20(m, 8H), 7.15-7.04(m, 4H), 6.85-6.82(m, 2H), 3.31-3.12(m, 4H), 2.65-2.42( m, 4H). 13 C NMR (75MHz, CDCl 3 ): δ 154.4, 147.7, 144.3, 143.5, 135.3, 133.8, 131.5, 129.4, 129.0, 127.6, 126.7, 122.4, 115.4, 60.3, 38.1, 31.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com