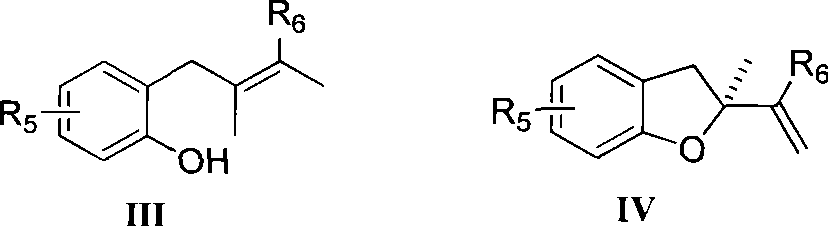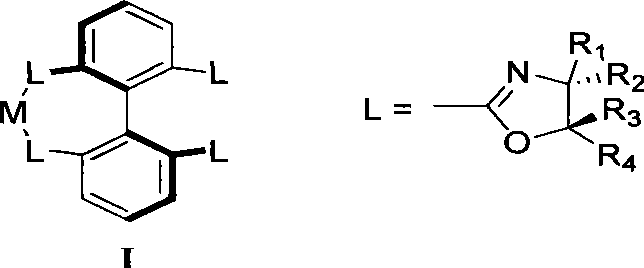Method for preparing chiral dihydrobenzofuran compounds and catalyst used thereby
A technology of chroman and compounds, applied in the field of a method and the catalyst used, can solve the problems of difficulty in obtaining optically pure axial chiral ligands, poor substrate applicability, complex separation methods, etc., and achieve a simple synthesis method , avoid waste of resources, high reactivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
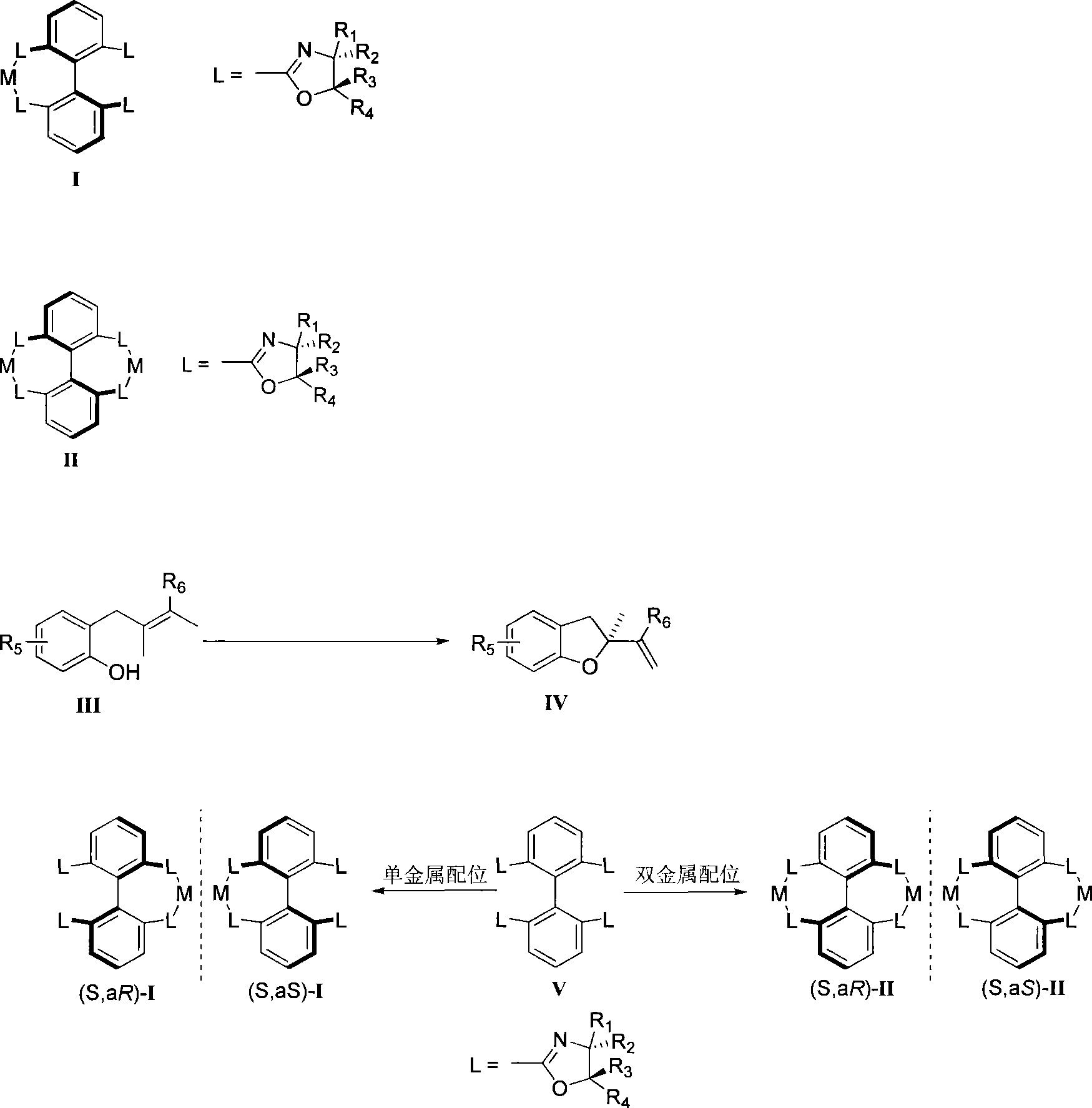
[0041] Embodiment 1: Compound (S, aS)-II(R 1 = Ph, R 2 = R 3 = R 4 =H, M=Pd) synthesis
[0042] Under nitrogen, V (7.4mg, 10.08□mol) was mixed with Pd(OCOCF 3 ) 2 (6.7 mg, 20.15 mol) was dissolved in tetrahydrofuran and stirred for 10 minutes to obtain a bimetallic axial chiral catalyst (S, aS)-II with a yield of 95%.
[0043] Its characteristic parameters are: 1 H NMR (400MHz, acetone-d 6 )δ7.90-8.00 (m, 6H, ArH), 7.35-7.39 (m, 12H, ArH), 7.01-7.04 (m, 8H, ArH), 5.11 (dd, J=7.2, 10.8Hz, 4H, OCH ), 4.88 (dd, J=9.2, 10.4Hz, 4H, NCH), 4.49 (dd, J=6.4, 9.6Hz, 4H, OCH).
Embodiment 2
[0044] Embodiment 2: Compound (S, aS)-II(R 1 =t-Bu,R 2 =R 3 =R 4 =H, M=Pd) synthesis
[0045] The preparation method of this embodiment is the same as that of Example 1.
[0046] The characterization parameters are: 1 H NMR (400MHz, acetone-d 6 ): δ8.32(d, J=3.6Hz, 4H, ArH), 8.18(dd, J=7.2, 8.4Hz, 2H, ArH), 4.53(t, J=9.2, 4H, NCH), 4.45(dd , J=5.2, 9.2Hz, 4H, OCH), 3.95(dd, J=5.6, 10.0Hz, 4H, OCH), 0.83(s, 36H, CH 3 ).
Embodiment 3
[0047] Embodiment 3: Compound (S, aS)-II(R 1 =i-Pr,R 2 =R 3 =R 4 =H, M=Pd) synthesis
[0048] The preparation method of this embodiment is the same as that of Example 1.
[0049] The characterization parameters are: 1 H NMR (400MHz, acetone-d 6 ): δ8.33(d, J=8.0Hz, 4H, ArH), 8.21(dd, J=7.2, 8.8Hz, 2H, ArH), 4.57(t, J=9.2, 4H, NCH), 4.26(dd , J=6.0, 8.8Hz, 4H, OCH), 3.65-3.71(m, 4H, OCH), 1.32(d, J=6.4Hz, 12H, CH 3 ), 1.03-1.12 (m, 4H, CH), 0.49 (d, J=6.8Hz, 12H, CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com