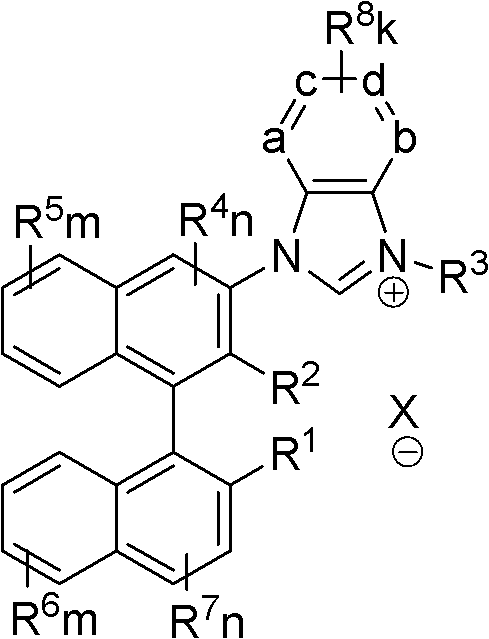
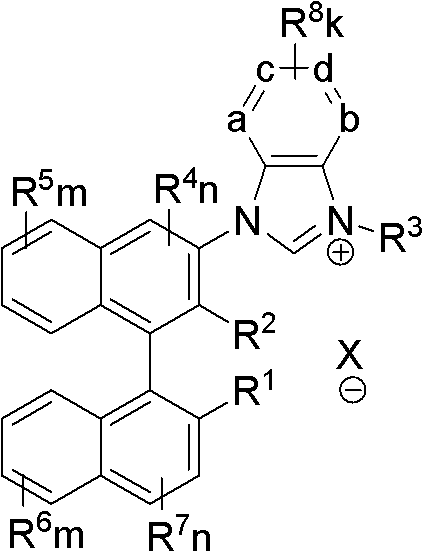
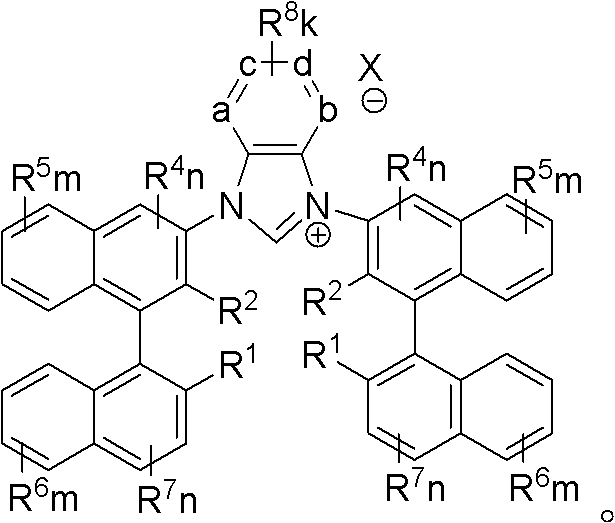
Axial chiral imidazole salt compound and preparation method thereof
An axial chirality and compound technology, applied in the field of chiral catalysts, can solve the problems of restricting the synthesis and application of new axial chiral azacarbenes, synthesis difficulties, chiral azacarbene synthesis difficulties, etc., and achieve high asymmetric selectivity , the effect of easy availability of raw materials and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1,3-bis{3-[2-methyl, 2′-hydroxy-((R)-(+)-1,1′-naphthyl)]}benzimidazole salt (IM-I-2) preparation:
[0042]
[0043] (1) Preparation of 3-halo-2-methyl, 2'-methoxymethyl ether-(R)-(+)-1,1'-binaphthalene (I-2): at room temperature, Dissolve 2-methyl, 2'-methoxymethyl ether-(R)-(+)-1,1'-binaphthalene (1.95g, 5mmol) in 80ml of ether, add butyllithium (4.17 ml, 2.4M), the reaction system reacted at room temperature for 4 hours. Afterwards, a tetrahydrofuran solution of iodine (2.54 g, 10 mmol dissolved in 20 ml tetrahydrofuran) was added at 0° C., and the reaction was continued at room temperature for 4 hours after the addition was completed. Thereafter, the reaction was extracted with saturated aqueous sodium sulfite solution, extracted three times with ethyl acetate, and finally washed with saturated brine. Afterwards, it was dried with anhydrous magnesium sulfate, separated by column chromatography, and the eluent was petroleum ether / ethyl acetate=20 / 1, and 3-halo-2...
Embodiment 2
[0049] 1,3-bis{3-[2-phenyl, 2′-hydroxyl-((R)-(+)-1,1′-naphthyl)]}benzimidazole salt (IM-I-1) preparation:
[0050]
[0051] 1,3-bis{3-[2-phenyl, 2′-hydroxyl-((R)-(+)-1,1′-naphthyl)]}benzimidazole salt (IM-I-1) The preparation takes 3-halo-2-phenyl, 2'-methoxymethyl ether-(R)-(+)-1,1'-binaphthalene (I-1) as raw material, and the remaining steps are the same as 1, Preparation method of 3-bis{3-[2-methyl, 2′-hydroxyl-((R)-(+)-1,1′-naphthalene)]}benzimidazolium salt (IM-I-2) Same, the final product (IM-I-1) yield of this embodiment is 63%. Its physical constants are: melting point is 321°C; [α] 20 D =+191.0 (measurement concentration c=0.1, measurement solvent is CH 2 Cl 2 ). 1 HNMR (DMSO, 400MHz): δ6.73 (2H, s), 7.06 (2H, d, 4Hz), 7.24-7.33 (14H, m), 7.44 (8H, s), 7.57-7.60 (2H, m), 7.67-7.73(2H, m), 7.99(2H, m), 8.13(2H, d, 8Hz), 8.21(2H, d, 8Hz), 8.44(2H, s), 9.74(2H, s), 10.79( 1H, s). 13 CNMR (DMSO, 100MHz): δ113.9, 122.0, 122.2, 123.9, 124.6, 124.7, 125.8, 126.0...
Embodiment 3
[0053] 1-phenyl, 3-{3-[2-methyl, 2′-hydroxyl-((R)-(+)-1,1′-naphthyl)]}benzimidazole salt (IM-II-1 ) preparation:
[0054]
[0055] (1)N 1 -[3-(2-phenyl, 2-methyl ether binaphthyl)]-1, the preparation of 2-o-phenylenediamine (III-1):
[0056] Under nitrogen protection, add a magnetic stir bar to a dry schlenk tube, add raw materials 2-phenyl, 2'-methoxymethyl ether, 3'-halo-(R)-(+)-1,1'- Add Pd(OAc) to binaphthyl (I-1) (516mg, 1.0mmol), ortho-phenylenediamine (108mg, 1.0mmol) 2 (22.5mg, 0.1mmol), BINAP (124.5g, 0.2mmol), Cs 2 CO 3 (626mg, 2.0mmol), 15ml solvent 1,4-dioxane, and the mixture was reacted at 100°C for 14 hours. After the reaction system was cooled to room temperature, the solid impurities in the system were filtered off, the filtrate was washed with saturated brine, dried with anhydrous sodium sulfate, and then separated by column chromatography, and the eluent was petroleum ether / ethyl acetate= 7 / 1. Finally, a yellow solid product was obtained with a yie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com