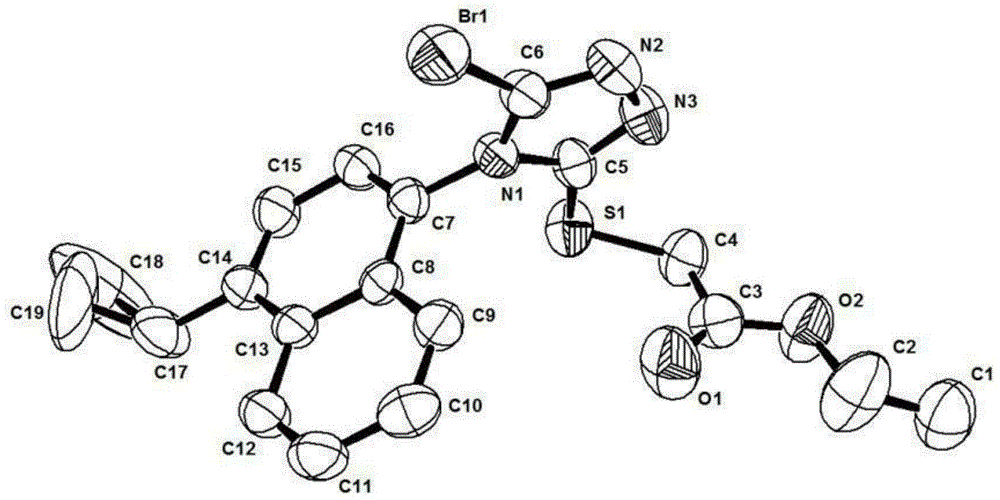
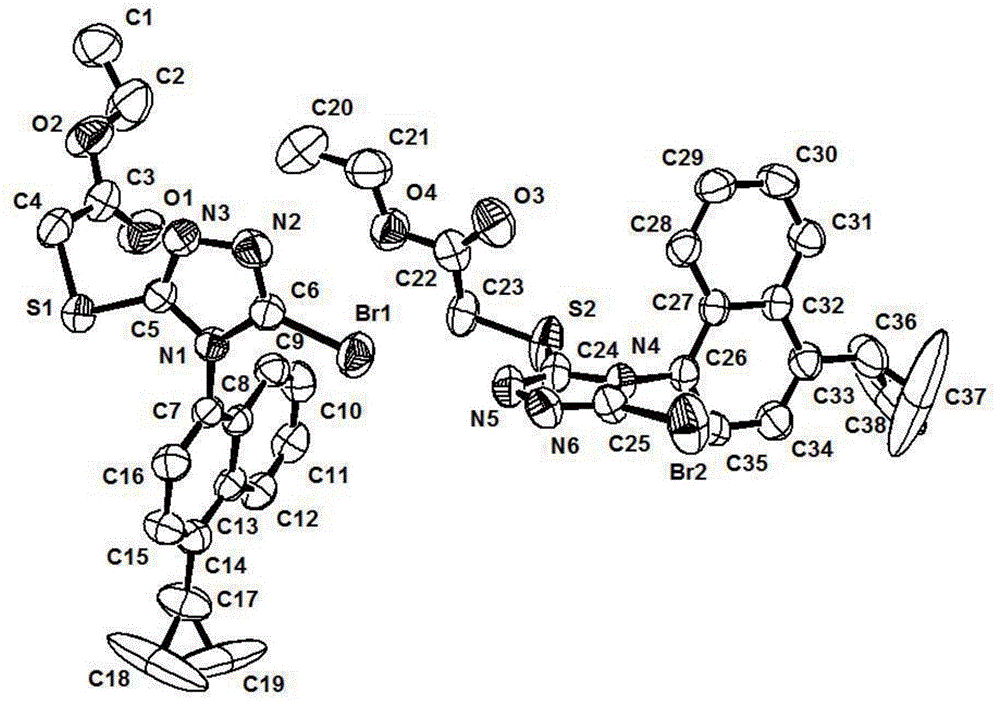
Axial chiral isomers and preparation method and pharmaceutical application thereof
An isomer and axial chirality technology, applied in the field of two axial chiral isomers and their pharmaceutically acceptable salts, can solve the problems of no specific reported separation technology, chiral synthesis technology, no explicit mention of Lesinurad, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1: the preparation of (-)-Lesinurad and (+)-Lesinurad
[0082]
[0083] synthetic route:
[0084]
[0085] Step 1: Synthesis of Compounds 12A and 12B
[0086] Compound 12 was synthesized according to the method reported in patent CN103524440A or patent W2009070740. Compound 12 (330.00mg, 763.31umol) was subjected to supercritical fluid chromatography SFC (chiral column: ChiralpakAS (250mm×30mm, 5um); mobile phase: supercritical CO 2 / ethanol (0.05% DEA)=70 / 30; flow rate: 60mL / min; detection wavelength: 220nm) to separate compound 12A (150.00mg, 346.96umol) and compound 12B (152.00mg, 351.58umol).
[0087] Compound 12A: 1 HNMR (400MHz, DMSO-d 6 )δ: 8.57(d, J=8.4Hz, 1H), 7.78-7.76(m, 1H), 7.69-7.58(m, 2H), 7.43(d, J=7.6Hz, 1H), 7.13(d, J =8.0Hz, 1H), 4.10-4.00(m, 4H), 2.59-2.51(m, 1H), 1.17-1.11(m, 5H), 0.89-0.83(m, 2H).SFC (chiral column: ChiralpakAS -H (250mm×4.6mm, 5um); mobile phase: ethanol (0.05% DEA) / supercritical CO 2 =5~40%; Flow rate: 2.5m...
Embodiment 2
[0119] Embodiment 2: the preparation of (-)-Lesinurad and (+)-Lesinurad
[0120]
[0121] synthetic route:
[0122]
[0123] Step 1: Synthesis of compound 14
[0124]Compound 13 (2.00g, 16.37mmol, 1.00eq) and pyridine (2.59g, 32.74mmol, 2.00eq) were dissolved in toluene (30.00mL), the mixture was cooled to 0°C in an ice-water bath, and 2-bromoethyl Acid chloride (3.09g, 19.64mmol, 1.20eq), the reaction solution was stirred at 0°C for 1 hour, and a large amount of white solid was precipitated. After the reaction was complete, it was filtered, the filter cake was washed with dichloromethane (3 mL), and the obtained filtrate was spin-dried to obtain a yellow oily crude product. The crude product was purified by column chromatography (eluent: 0-15% ethyl acetate / petroleum ether) to obtain compound 14 (3.20 g, 13.16 mmol, yield 80.41%) as a colorless oil. 1 HNMR (400MHz, CDCl 3 )δ: 7.45-7.22 (m, 6H), 6.00-5.92 (m, 1H), 4.07 (s, 1H), 3.85 (s, 1H), 1.63-1.55 (d, J=4Hz, 3H)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com