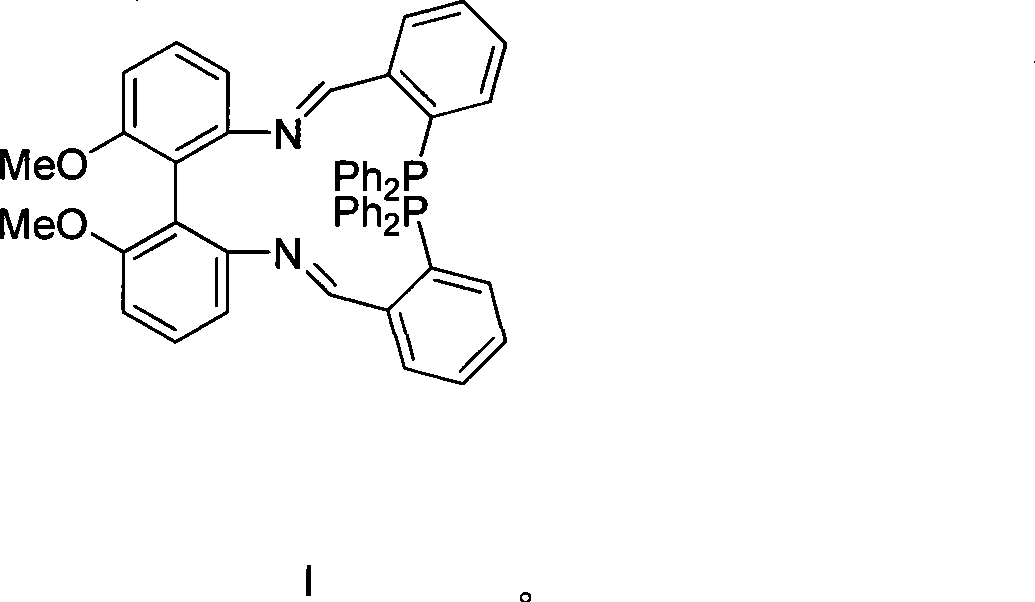
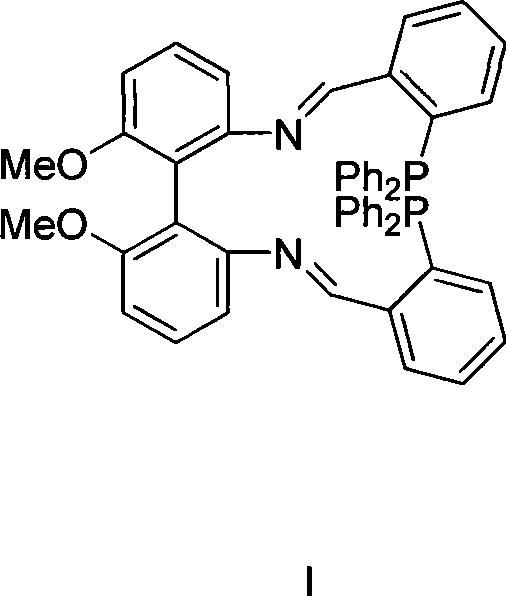
Axial chirality diphosphine ligand containing bis-schiff base
A technology of bis-Schiff base and bis-phosphine ligand, which is applied in the chemical field of chemical technology, can solve the problems of large steric hindrance, limit the rotation angle of biphenyl, etc., and achieve the effect of high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
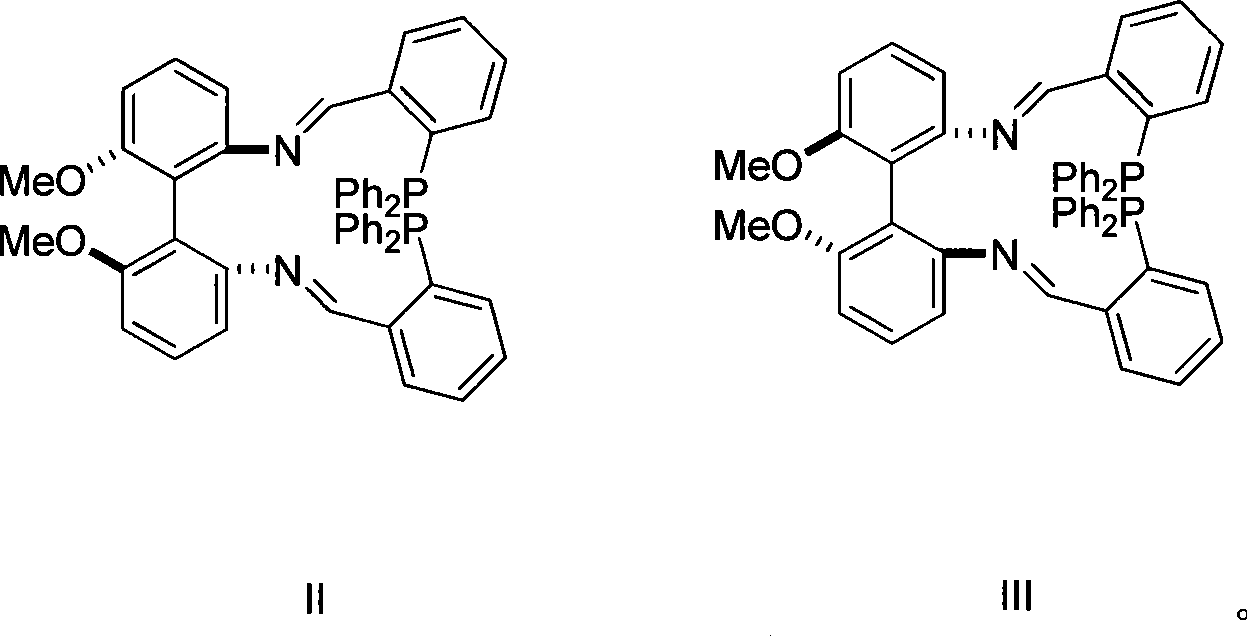
[0017] (1) Preparation of Compound II from Compound IV
[0018] Compound IV (1.0g, 4.1mmol) and 2-diphenylphosphinebenzaldehyde (2.38g, 8.2mmol) were refluxed in toluene for fourteen hours, and purified by column chromatography to obtain the product II ( 2.9 g, 89%).
[0019]
[0020] 1 H NMR (CDCl 3 400, MHz) δ 8.93(d, 2H, N=CH, J=5.6Hz), 7.78-6.69(m, 32H, ArH), 6.35(d, 2H, ArH), 3.82(s, 6H, OCH 3 ) 31 P NMR (CDCl 3 , 161MHz) δ-14.17
Embodiment 2
[0022] (1) Preparation of Compound III from Compound V
[0023] Compound V (2.0g, 8.2mmol) and 2-diphenylphosphinebenzaldehyde (4.76g, 16.4mmol) were refluxed in toluene for fourteen hours, and purified by column chromatography to obtain product III ( 5.7 g, 87%).
[0024]
[0025] 1 H NMR (CDCl 3 400, MHz) δ 8.93(d, 2H, N=CH, J=5.6Hz), 7.78-6.69(m, 32H, ArH), 6.35(d, 2H, ArH), 3.82(s, 6H, OCH 3 ) 31 P NMR (CDCl 3 , 161MHz) δ-14.18
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com