Synthetic method of pyraclostrobin intermediate pyrazole alcohol
A technology of pyraclostrobin and pyraclostrobin, which is applied in the synthesis process field of pyraclostrobin intermediate pyraclostrobin, can solve the problems of unfavorable economy, unfavorable economy, low yield, etc., and achieve Effects of optimizing synthesis process design, improving economic benefits, and saving synthesis costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
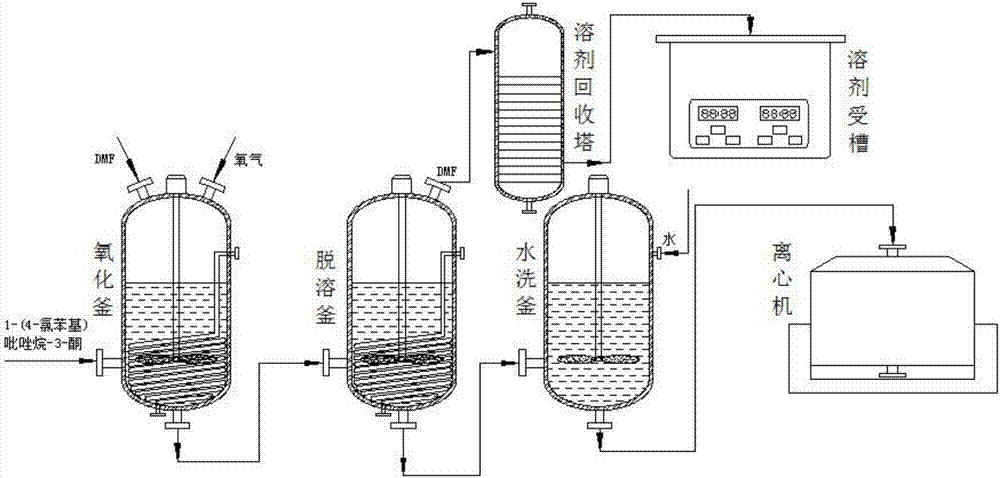
[0028] see figure 1 As shown, the technical scheme adopted in the present invention is: a kind of synthesis process of pyraclostrobin intermediate pyrazol, and the specific process method includes the following steps:
[0029] (1). Oxidation: Put 1-(4-chlorophenyl)pyrazolidin-3-one into the oxidation reaction kettle, add the solvent DMF from the top side of the oxidation reaction kettle, and continue to pass through the top side of the oxidation reaction kettle. Enter oxygen for a period of time, use a stirrer to stir, keep warm in the oxidation reaction kettle for 1 hour, and transfer to the precipitation kettle after the oxidation reaction is completed;
[0030] (2). Precipitation: transfer the organic solution in the oxidation reaction kettle containing DMF into the precipitation kettle, use a stirrer to stir in the precipitation kettle, heat and evaporate the DMF, and transfer the DMF that evaporates to form steam into the solvent The recovery tower is used for recovery, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com