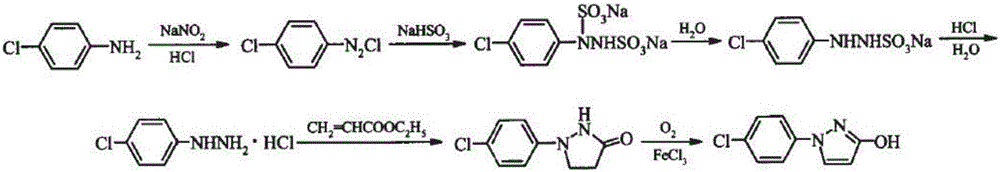
Synthetic process of 1-(4-chlorophenyl)-3-hydroxy-1-h-pyrazole
A synthesis process, a chlorophenyl technology, is applied in the field of synthesis technology of 1--3-pyrazolol, can solve the problems of material waste, product yield, cumbersome reaction, decrease and the like, and achieves the effects of reducing production and consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0016] The synthetic technique of 1-(4-chlorophenyl)-3-pyrazolol is characterized in that, synthetic technique comprises the following steps: (1) in the diazotization still that material is glass-lined, drop into the p-chlorine of 1 weight part Aniline, the hydrochloric acid of 2.5 parts by weight and the water of 1.5 parts by weight, open the anchor type stirring device, control the peripheral speed of the anchor type stirring paddle outer edge to be 1.5m / s, increase the reaction temperature, continue to stir and make solid all dissolve, control Temperature is 70 DEG C, and the pressure is maintained as slight positive pressure. After the reaction is completed, slowly dropwise add 3 parts by weight of sodium nitrite solution with a concentration of 10%, and monitor the reaction with HPLC. After the reaction, the next step is directly carried out without processing; ) Add 1 weight part of sodium sulfite to the product of step 1, stir evenly, raise the reaction temperature to 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com