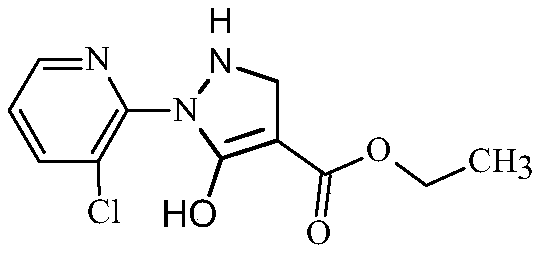
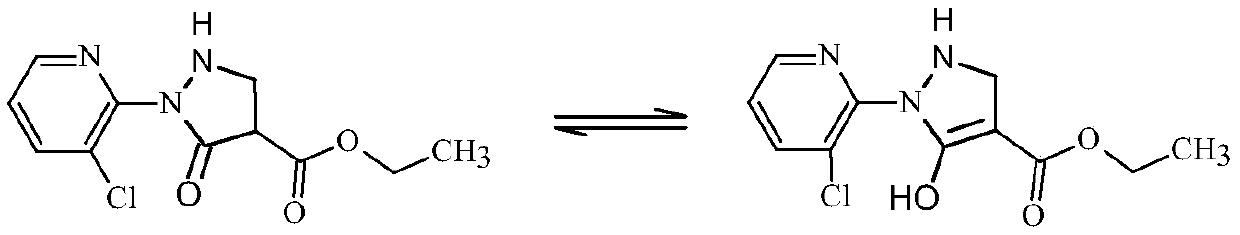Synthesis method of 2-(3-chloropyridine-2-yl)-5-hydroxy-3-pyrazolidine ethyl formate
A kind of technology of ethyl pyrazolidine carboxylate and synthetic method, which is applied in the field of synthesis of ethyl 2--5-hydroxy-3-pyrazolidine carboxylate, can solve the problems of low yield and achieve safe and reliable synthesis without difficulty The effect of mild decomposition and reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0026] A 1000ml four-necked bottle with a stirring device is installed in a water bath, and the water bath is filled with iced brine. Add 100.5 g of glacial acetic acid (98%, 1.64 mol) and 250 g of n-butanol to the four-neck flask, stir, and add 81.2 g of 3-chloro-2-aminopyridine (98.3%, 0.621 mol). Another 45.6g (98.5%, 0.651mol) of sodium nitrite was dissolved in 57g of water, added to the constant pressure funnel, and connected to the above-mentioned four-necked bottle. When the temperature in the four-necked bottle drops to -2~-5°C, add the sodium nitrite solution in the constant pressure funnel dropwise into the four-necked bottle. Control the temperature in the bottle between -5 and 10°C. After the dropwise addition, keep warm at about 0°C. Check that the content of 3-chloro-2-aminopyridine is <0.5%. After the reaction is over, add about 1.8g of urea to starch-KI The test paper does not change color.
example 2
[0028] A 1000ml four-necked bottle with a stirring device is installed in a water bath, and the water bath is filled with iced brine. 109.5 g (98.8%, 0.621 mol) of diethyl succinate was added to the four-necked flask. Get two constant pressure funnels and add 30% ammonia water and the diazo solution of Example 1 respectively, and connect to the four-necked bottle. When the temperature in the four-necked bottle drops below 0°C, add ammonia water and diazo solution to the four-necked bottle at the same time, keep the temperature in the four-necked bottle at -5-15°C, and pH 4-6.5, keep warm after the addition Insulate at 0-15°C, take samples, after HPLC detects that the diazonium salt disappears, change to water bath heating, add ammonia water dropwise to pH 9-10.5, keep at 30-95°C for 2 hours, cool down to room temperature, add glacial acetic acid and to pH 6.5. Transfer the material in the four-necked bottle to a separatory funnel and let it stand for one hour. The organic p...
example 3
[0030] A 1000ml four-necked bottle with a stirring device is installed in a water bath, and the water bath is filled with iced brine. Add 101.2g (98%, 1.65mol) of glacial acetic acid and 250g of n-butanol to the four-necked flask, stir, and add 81.3g (98.3%, 0.622mol) of 3-chloro-2-aminopyridine. Another 82.2 g (82.0%, 0.654 mol) of n-butyl nitrite in n-butanol was added to the constant pressure funnel. When the temperature in the four-necked bottle drops to -2~-5°C, add n-butyl nitrite in the constant pressure funnel dropwise into the four-necked bottle. Control the temperature in the bottle between -5 and 10°C. After the dropwise addition, keep warm at about 0°C. Check that the content of 3-chloro-2-aminopyridine is <0.5%. After the reaction is over, add about 1.9g of urea to starch-KI The test paper does not change color.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com