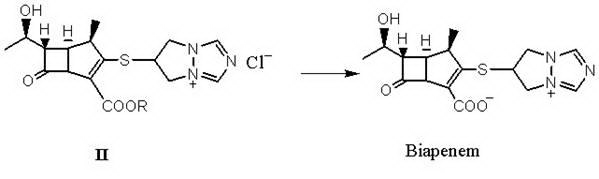
Preparation method of biapenem
A technology of biapenem and triphenylphosphine, applied in the field of preparation of biapenem, can solve the problems of danger, inconvenience in production, easy occurrence of danger and the like, and achieve the effects of improving safety, simple operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
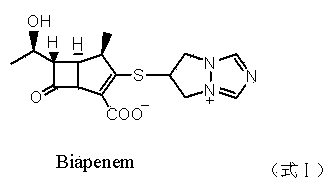
[0027] Add 150 g of 1R,5S,6S)-2-[(6,7-dihydro-5H-pyrazole-[l,2-α][1,2,4]triazole ylide chloride to the reaction flask -6 base) sulfur]-6-[(1R)-1-hydroxyethyl]-1-methylcarbapenem-2-ene-3-carboxylic acid allyl ester (formula III compound), add 500ml tetrahydrofuran, Add 3.0 g of triphenylphosphine and 2.8 g of tetrakis(triphenylphosphine) palladium, stir at room temperature for 2 hours, add water to dilute, wash with dichloroethane, and concentrate under reduced pressure to obtain an aqueous solution. Then adjust the solution to Ph=5.5 with O.1N HCl, then add acetone, stir and crystallize at 10°C for 2 hours, and filter to obtain 52 g of off-white powder biapenem, with a yield of 65%.
[0028] Colorless crystal, melting point 210~218°C (decomposition).
[0029] 1 HNMR (D 2 0,300MHz)d; 1.26(d,3H,J=7.3Hz),1.30(d,3H,J=6.6Hz),
[0030] 3.41 (dq, 1H, J=7.3, 9.6Hz), 3.53 (dd, 1H, J=3.0, 5.9Hz), 4.27 (dq, 1H, J=5.9, 6.6Hz), 4.31 (dd, 1H, J=3.0, 9.6Hz), 4.71-4.80(m, 2H), 4.98(m, 1H...
Embodiment 2
[0032] Add 65 g of 1R,5S,6S)-2-[(6,7-dihydro-5H-pyrazole-[l,2-α][1,2,4]triazole ylide chloride to the reaction flask -6 base) sulfur]-6-[(1R)-1-hydroxyethyl]-1-methylcarbapenem-2-ene-3-carboxylic acid allyl ester (compound of formula III), add 200ml ethyl acetate Add 1.3 g of triphenylphosphine and 1.2 g of tetrakis(triphenylphosphine) palladium to the ester, stir and react at room temperature for 1.5 h, add water to dilute, wash with dichloromethane, and concentrate under reduced pressure to obtain an aqueous solution. Then adjust the pH of the solution to 6.0 with O.1N HCl, then add acetone, stir and crystallize at -5°C for 3 hours, and filter to obtain 24 g of biapenem with a yield of 70%. Colorless crystal, melting point 210~218°C (decomposition).
Embodiment 3
[0034] Add 30 g of 1R,5S,6S)-2-[(6,7-dihydro-5H-pyrazole-[l,2-α][1,2,4]triazole ylide chloride to the reaction flask -6 base) sulfur]-6-[(1R)-1-hydroxyethyl]-1-methylcarbapenem-2-ene-3-carboxylic acid allyl ester (compound of formula I), add 200ml dichloro Add 1.3 g of triphenylphosphine and 0.5 g of tetrakis(triphenylphosphine) palladium to methane, stir and react at room temperature for 2 h, add water to dilute, wash with dichloromethane, and concentrate under reduced pressure to obtain an aqueous solution. Then adjust the solution to Ph=5.0 with O.1N HCl, then add acetone, stir and crystallize at 15°C for 2 hours, and filter to obtain 10 g of biapenem with a yield of 63%. Colorless crystal, melting point 210~218°C (decomposition).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com