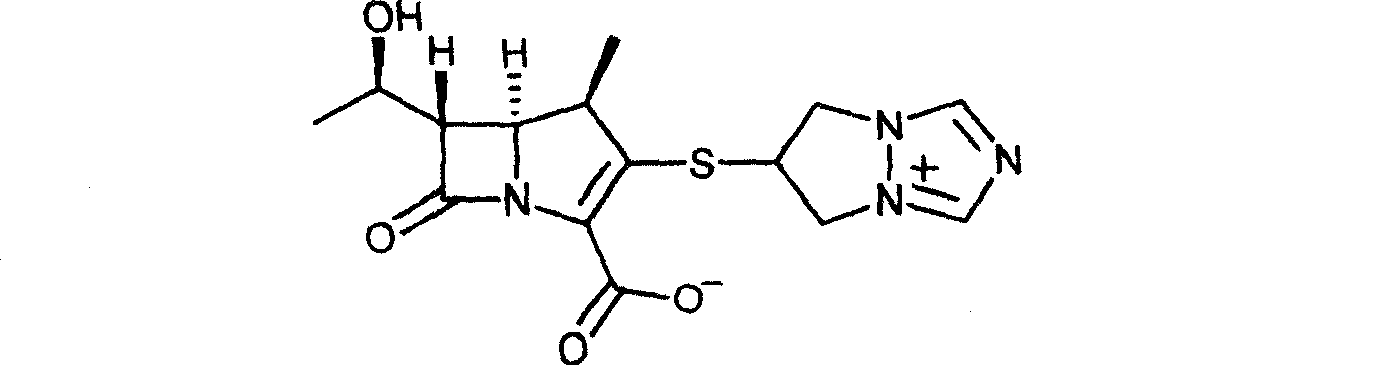
Improved Biapenem preparation method
A technology of biapenem and dihydrogen, which is applied in the field of preparation of biapenem, can solve the problems of low yield, need of column chromatography, and incapability of large-scale production, and achieve the effect of low-cost and high-efficiency synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] In the following section a preferred embodiment is illustrated by illustrating an example of the method of the invention. But it is in no way intended to limit the scope of the invention.
[0026] Preparation of Biapenem Example
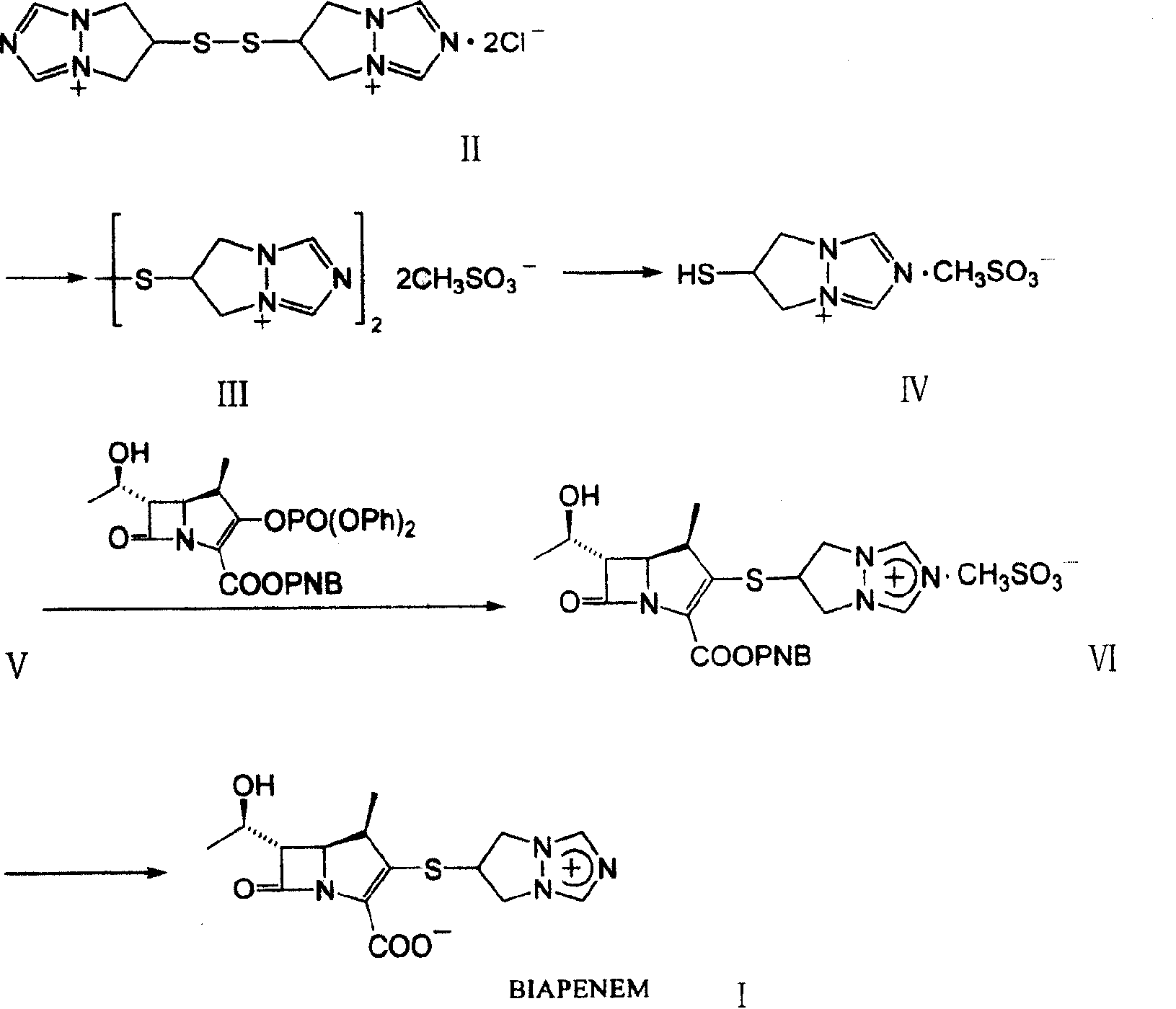
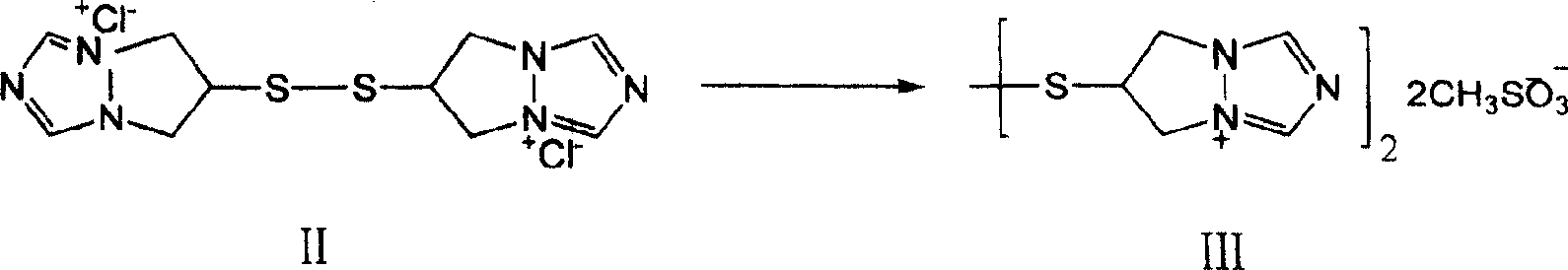
[0027] Step 1 Preparation of bis(6,7-dihydro-5H-pyrazolo[1,2a][1,2,4]triazolium-6-yl)disulfide dimesylate
[0028]
[0029] Dissolve 8 g of bis(6,7-dihydro-5H-pyrazolo[1.2a][1.2.4]triazolium-6-yl)disulfide dichloride (II) in 20 ml of methanol, 5 ml of methanesulfonic acid was added under stirring at room temperature, and 7.5 ml of acetone was added to the residue concentrated under reduced pressure for crystallization to obtain 7.8 g of off-white solid.
[0030] Step 2 Preparation of 6,7-dihydro-6-mercapto-5H-pyrazolo[1,2a][1,2,4]triazolium mesylate
[0031]
[0032] The product in step 1 was off-white solid, bis(6,7-dihydro-5H-pyrazolo[1,2-a][1,2,4]triazol-6-yl)disulfide disulfide Add 6g of mesylate (III), 30ml of tetrahydrofuran an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com