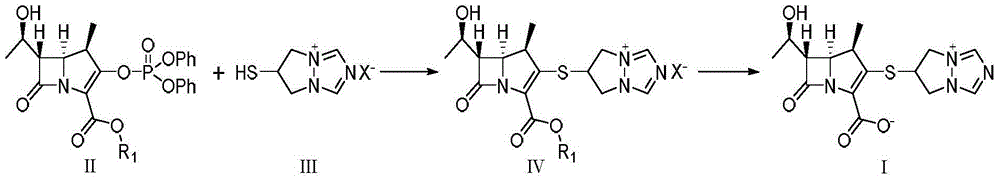
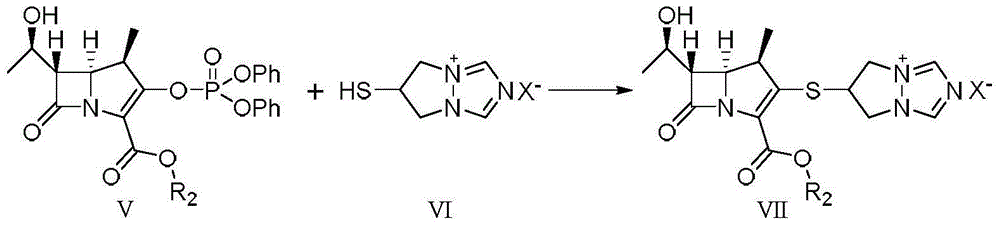
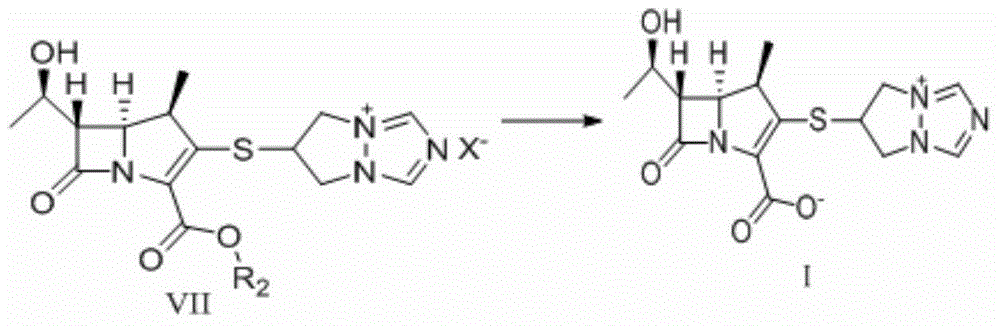
Preparation method of high-purity biapenem
A biapenem, high-purity technology, applied in the field of preparation of pharmaceutical compounds, can solve the problems of reduced purity, difficult separation, difficult recovery, etc., and achieve the effects of reducing the generation of impurities, small reaction volume, and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 6-[[(4R,5S,6S)-2-[(4-nitrobenzyloxy)carbonyl]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo- 1-Azabicyclo[3.2.0]hept-2-en-3-yl]thio]-6,7-dihydro-5H-pyrazolo[1,2-a][1,2,4]tri Preparation of azol-4-ium chloride (compound of formula VII)
[0044] (4R,5R,6S)-3-(Diphenoxyphosphonooxy)-6-((R)-1-hydroxyethyl)-4-methyl-7-carbonyl-1-azabicyclo [3.2.0] p-nitrobenzyl hept-2-ene-2-carboxylate (compound of formula V) 50.0g, 6-mercapto-6,7-dihydro-5H-pyrazolo[1,2-a Add 18.6g of [1,2,4]triazol-4-ium chloride (compound of formula VI), 23.7g of N,N-dimethylformamide and 11.7g of triethylamine into 400g of acetonitrile, stir and blow nitrogen Protected, stirred at -10 to -15°C for 10 hours, filtered, and dried to give 6-[[(4R,5S,6S)-2-[(4-nitrobenzyloxy)carbonyl]-6-[(1R)- 1-Hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-en-3-yl]thio]-6,7-dihydro-5H-pyridine Azolo[1,2-a][1,2,4]triazol-4-ium chloride (compound of formula VII) 36.7g, yield 83.7%, purity 98.6%.
Embodiment 2
[0046] 6-[[(4R,5S,6S)-2-[(4-nitrobenzyloxy)carbonyl]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo- 1-Azabicyclo[3.2.0]hept-2-en-3-yl]thio]-6,7-dihydro-5H-pyrazolo[1,2-a][1,2,4]tri Preparation of azol-4-ium chloride (compound of formula VII)
[0047] (4R,5R,6S)-3-(Diphenoxyphosphonooxy)-6-((R)-1-hydroxyethyl)-4-methyl-7-carbonyl-1-azabicyclo [3.2.0] p-nitrobenzyl hept-2-ene-2-carboxylate (compound of formula V) 50.0g, 6-mercapto-6,7-dihydro-5H-pyrazolo[1,2-a ][1,2,4]Triazol-4-ium chloride (compound of formula VI) 19.2g, N,N-dimethylformamide 23.7g and triethylamine 11.7g were added to 400g of acetonitrile, stirred, nitrogen gas Protected, stirred at -10 to -15°C for 10 hours, filtered, and dried to give 6-[[(4R,5S,6S)-2-[(4-nitrobenzyloxy)carbonyl]-6-[(1R)- 1-Hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-en-3-yl]thio]-6,7-dihydro-5H-pyridine Azolo[1,2-a][1,2,4]triazol-4-ium chloride (compound of formula VII) 39.1g, yield 89.1%, purity 99.0%.
Embodiment 3
[0049] 6-[[(4R,5S,6S)-2-[(4-nitrobenzyloxy)carbonyl]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo- 1-Azabicyclo[3.2.0]hept-2-en-3-yl]thio]-6,7-dihydro-5H-pyrazolo[1,2-a][1,2,4]tri Preparation of azol-4-ium chloride (compound of formula VII)
[0050] (4R,5R,6S)-3-(Diphenoxyphosphonooxy)-6-((R)-1-hydroxyethyl)-4-methyl-7-carbonyl-1-azabicyclo [3.2.0] p-nitrobenzyl hept-2-ene-2-carboxylate (compound of formula V) 400.0g, 6-mercapto-6,7-dihydro-5H-pyrazolo[1,2-a Add 154.8g of [1,2,4]triazol-4-ium chloride (compound of formula VI), 129.6g of N,N-dimethylformamide and 93.6g of triethylamine into 3200g of acetonitrile, stir, and blow nitrogen Protected, stirred at -10 to -15°C for 8 hours, filtered, and dried to obtain 6-[[(4R,5S,6S)-2-[(4-nitrobenzyloxy)carbonyl]-6-[(1R)- 1-Hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-en-3-yl]thio]-6,7-dihydro-5H-pyridine Azolo[1,2-a][1,2,4]triazol-4-ium chloride (compound of formula VII) 320.3g, yield 91.2%, purity 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com