Folate-targeted reduction sensitive drug-carrying polymer nano-micelle as well as preparation method and application thereof
A drug-loaded polymer and folic acid targeting technology, which is applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve the problems of tumor cell recurrence or metastasis, uneven heat distribution, and difficulty in killing cancer cells, and achieve the goal of overcoming In vivo instability and ease of clearance, prolongation of circulation time, effects of long circulation properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of PCL-ss-PEG-ss-PCL.
[0044] A synthesis method of reduction-sensitive amphiphilic triblock copolymer (PCL-ss-PEG-ss-PCL) based on PCL 3750 -ss-PEG 7500 -ss-PCL 3750 The synthetic method of is example, comprises the following steps:
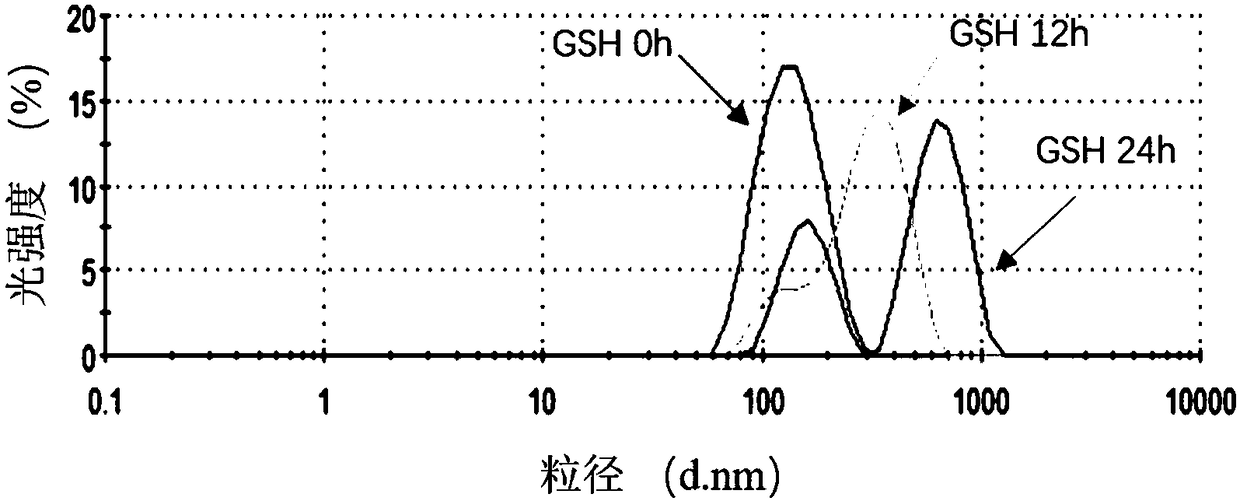
[0045] (1) Accurately weigh 1g of Py-ss-PEG 7500 -ss-Py (purchased from Beijing Jiankai Technology Co., Ltd.) was dissolved in 12 mL of dimethyl sulfoxide (DMSO). After fully dissolved, add 25 μL mercaptoethanol and 20 μL acetic acid. The reaction was carried out under magnetic stirring at 25 °C for 24 h. After the reaction, the reaction mixture was transferred to a dialysis bag of MWCO8000-14000Da, and placed in distilled water for dialysis for 24 h. Then lyophilized to obtain pure white powder product HO-SS-PEG 7500 -SS-OH.
[0046] (2) Accurately add 1 g of purified ε-caprolactone monomer and 1 g of HO-ss-PEG 7500 -ss-OH In the polymerization tube, add a drop of stannous octoate as a catalyst. The reaction system w...
Embodiment 2
[0054] A method for preparing reduction-sensitive polymer micelles (DINPs) co-encapsulating DOX and ICG, comprising the following steps:
[0055] (1) First transfer doxorubicin hydrochloride and ICG to hydrophobicity respectively: add 1% (mass percentage of material) doxorubicin hydrochloride to methanol solution containing triethylamine (20 μL triethylamine / 1 mg doxorubicin) , react at room temperature for 20 hours under magnetic stirring; mix ICG and tetrabutylammonium iodide (1:5.72 mass ratio) in dichloromethane, and react at room temperature for 20 hours under magnetic stirring.
[0056] (2) Fully dissolve PCL-ss-PEG-ss-PCL in the organic solvent dichloromethane, then add desalted doxorubicin and transhydrophobic ICG solution and mix well, remove the organic solvent with a rotary evaporator, and make it in eggplant-shaped A uniform film is formed on the inner wall of the bottle, and the residual dichloromethane is blown dry with nitrogen, and placed in a vacuum drying ove...
Embodiment 3
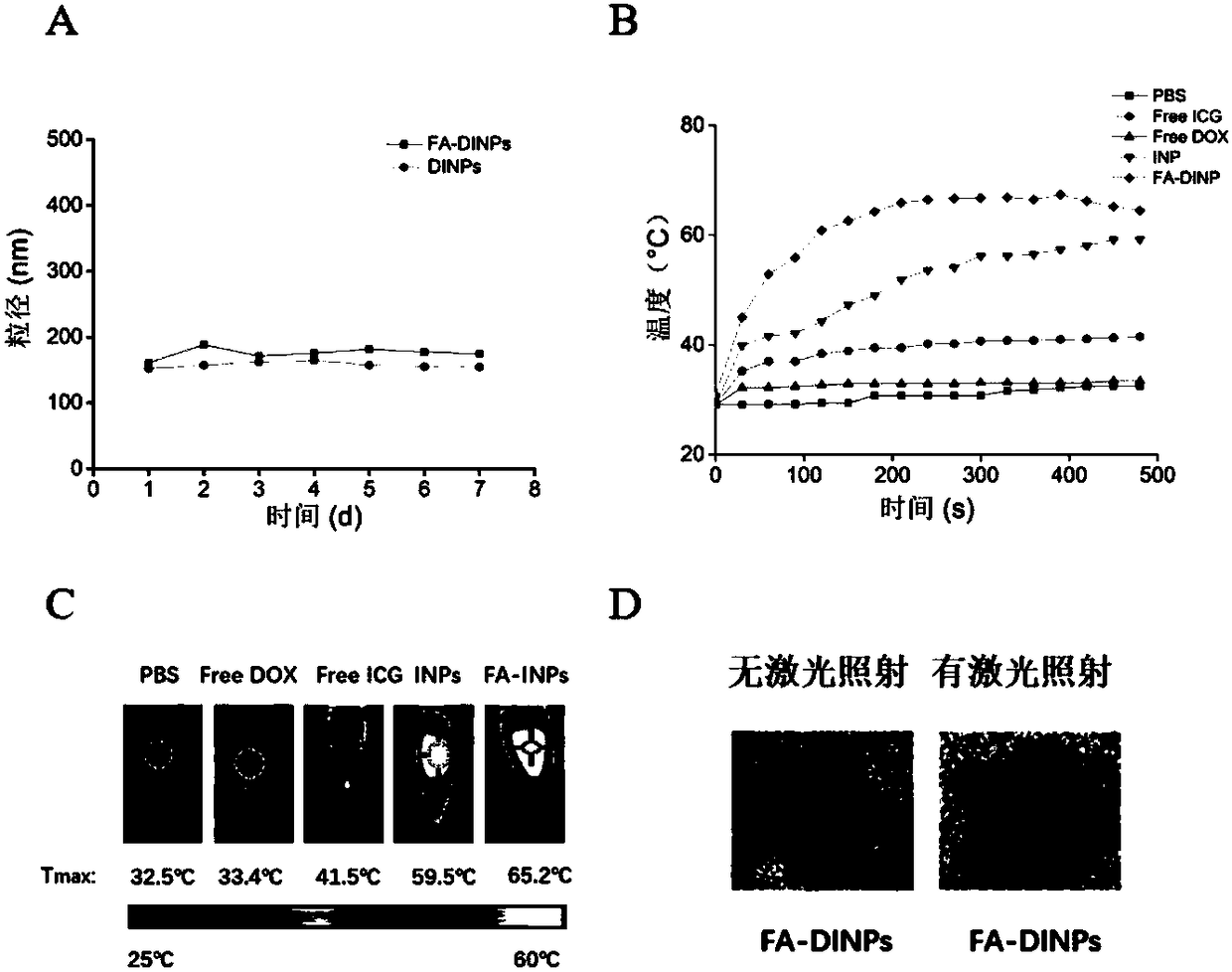
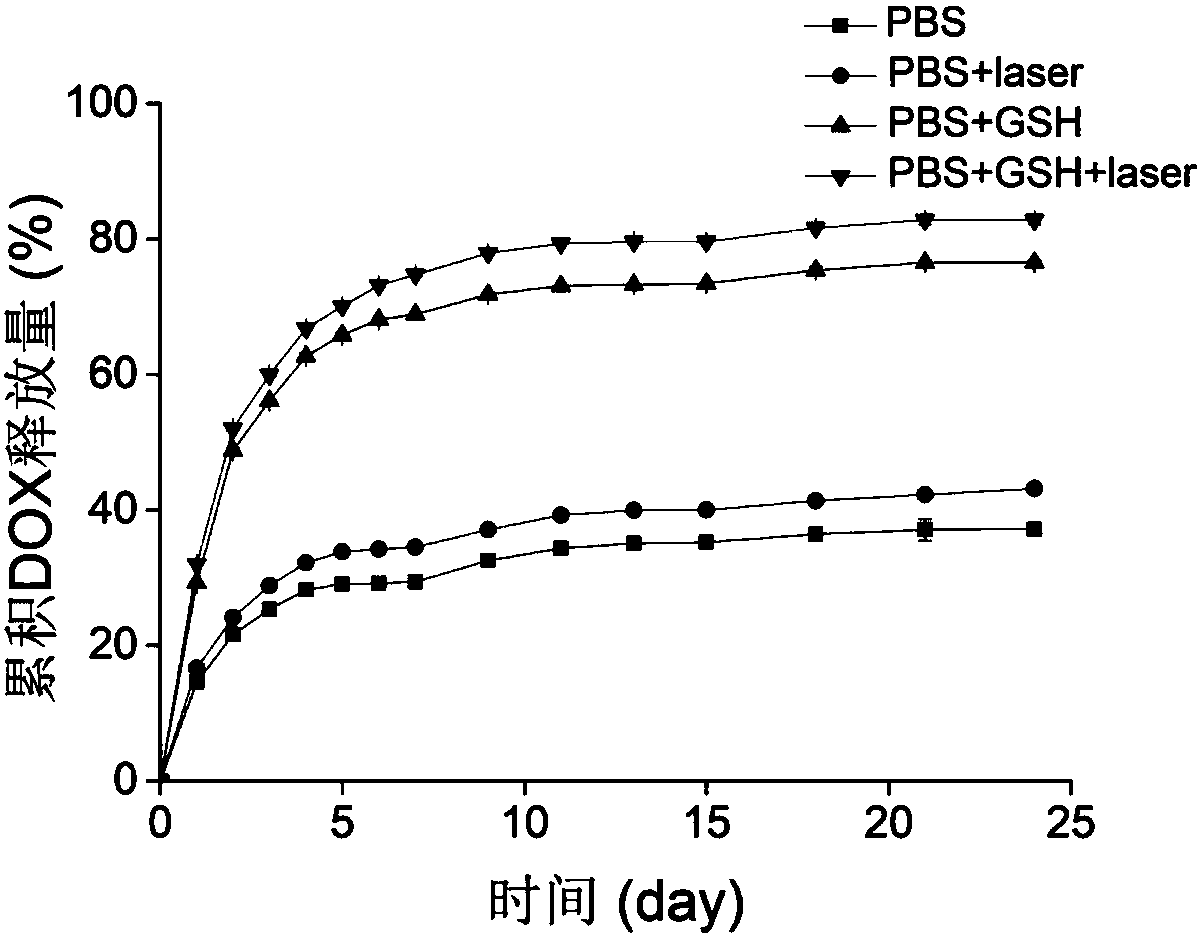
[0074] Study on drug release of FA-DINPs in vitro
[0075] The samples prepared in (3) of Example 2 were divided into 12 groups and released in MWCO 8000-14000Da dialysis bags. The release liquids were PBS (pH 7.4) and 10mM GSH PBS (pH 7.4). The release liquids were 20 mL, placed on a shaker, and shaken at 37°C at 120 rpm. After a certain period of time, all the release liquids were taken out to measure the drug content, and the same volume was added. of fresh release fluid. The laser irradiation group needs to use 1 W / cm before putting into the dialysis bag 2 The 808 nm laser was pre-irradiated for 5 min.
[0076] see release result image 3 , indicating that the release of doxorubicin in FA-DINPs was affected by the reduction of the release fluid and laser irradiation. After 2 days of incubation, FA-DINPs released 21.59 % and 48.77 % of doxorubicin in PBS (pH 7.4) and 10 mM GSH PBS (pH 7.4), respectively; The DOX released in PBS (pH 7.4) and 10mM GSH PBS (pH 7.4) were 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com