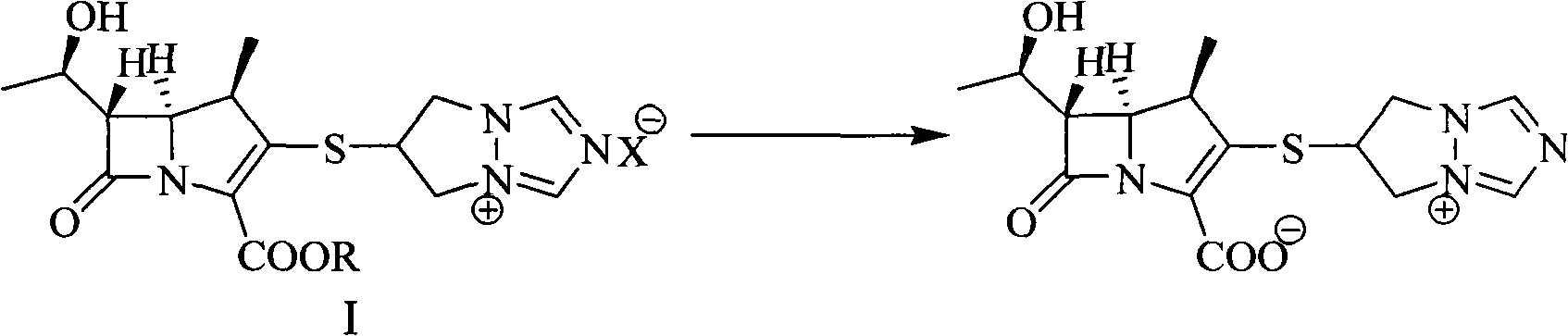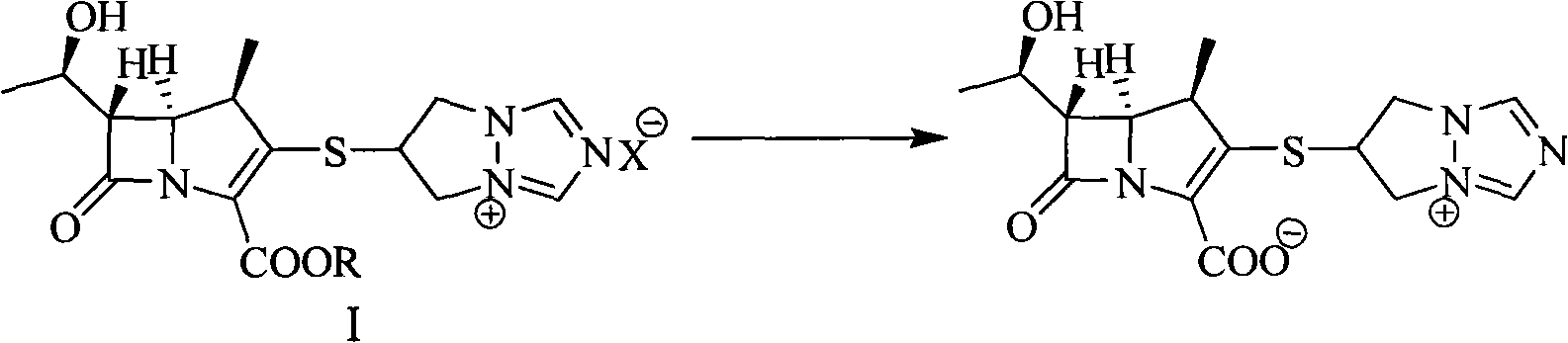Method for preparing biapenem with high purity
A technology of biapenem and synthesis method, which is applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of lower than 30% yield, low yield, expensive and other problems, and achieves high product purity and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 10.0g (19.2mmol) 6-[[(4R,5S,6S)-2-(benzyloxycarbonyl)-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo -1-Azabicyclo[3,2,0]hept-2-en-3-yl]thio]-6,7-dihydro-5H-pyrazolone[1,2-α][1,2, 4] Triazol-4-ium chloride, add 150mL tetrahydrofuran, 150mL N-methylmorpholine / acetic acid buffer (pH=5.5), stir to dissolve, add 6.0g Raney Ni, at 20°C, 1.2MPa hydrogen pressure Under hydrogenation 2h. Filter, wash the filter cake with 10 mL of distilled water, wash the filtrate twice with 150 mL of ethyl acetate, then add dropwise 500 mL of ethanol, stir at -10°C for 2 hours, filter, wash the solid with 40 mL of ethanol twice, and dry under reduced pressure to obtain bia Penem 5.2g (yield: 77.5%, purity: 99.53%).
Embodiment 2
[0026] 10.0g (19.2mmol) 6-[[(4R, 5S, 6S)-2-(4-nitrobenzyloxycarbonyl)-6-[(1R)-1-hydroxyethyl]-4-methyl- 7-Oxo-1-azabicyclo[3,2,0]hept-2-en-3-yl]thio]-6,7-dihydro-5H-pyrazolone[1,2-α][ 1,2,4] Triazol-4-ium chloride, add 150mL tetrahydrofuran, 150mL N-methylmorpholine / acetic acid buffer solution (pH=5.5), stir to dissolve, add 5.0g Raney Ni, at 20°C, Hydrogenation under 1.2MPa hydrogen pressure for 2h. Filter, wash the filter cake with 10 mL of distilled water, wash the filtrate twice with 150 mL of ethyl acetate, then add dropwise 500 mL of ethanol, stir at -10°C for 2 hours, filter, wash the solid with 40 mL of ethanol twice, and dry under reduced pressure to obtain bia Penem 5.0 g (yield: 74.5%, purity: 99.73%).
Embodiment 3
[0028] 10.0g (19.2mmol) 6-[[(4R, 5S, 6S)-2-(4-nitrobenzyloxycarbonyl)-6-[(1R)-1-hydroxyethyl]-4-methyl- 7-Oxo-1-azabicyclo[3,2,0]hept-2-en-3-yl]thio]-6,7-dihydro-5H-pyrazolone[1,2-α][ 1,2,4] Triazol-4-ium methanesulfonate, add 150mL tetrahydrofuran, 150mL N-methylmorpholine / acetic acid buffer (pH=5.5), stir to dissolve, add 5.0g Raney Ni, at 20 ℃, hydrogenation under 1.2MPa hydrogen pressure for 2h. Filter, wash the filter cake with 10 mL of distilled water, wash the filtrate twice with 150 mL of ethyl acetate, then add dropwise 500 mL of ethanol, stir at -10°C for 2 hours, filter, wash the solid with 40 mL of ethanol twice, and dry under reduced pressure to obtain bia Penem 4.6g (yield: 71.6%, purity: 99.65%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com