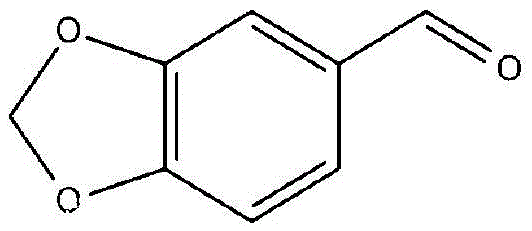
The synthetic method of 3,4-methylenedioxybenzaldehyde
A technology of methylene dioxybenzaldehyde and a synthetic method, applied in directions such as organic chemistry, can solve the problems of long process time, expensive raw and auxiliary materials, complicated process, etc., and achieves stable process quality, complete conversion of raw materials, and process steps. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 3m 3 Add 245kg of 50% glyoxylic acid aqueous solution to the enamel reaction kettle, then cool down to 10°C, add 265kg of 45% sulfuric acid aqueous solution, control the system temperature not more than 20°C, continue to cool down to 5°C after adding, and then transfer to 3M 3 Stainless steel reaction kettle, add 150kg of catechol methylene ether, start stirring, control the reaction temperature at about 5°C, continue to react for 3-4 hours after adding catechol methylene ether, then add 500kg of water to terminate the reaction; add water and continue Stir for 15min, then put the material into the 5m 3 Enamel reaction kettle, static phase separation, water phase separation, solid phase pumped into 5m 3 Add 100kg of 27% hydrochloric acid, 600kg of toluene, 20kg of catalyst zinc chloride, and start adding hydrogen peroxide (220kg) dropwise when the temperature rises to 65°C. Stop stirring, let stand to separate the phases, separate the water phase to recover the catalys...
Embodiment 2
[0039] 3m 3Add 245kg of 50% glyoxylic acid aqueous solution to the enamel reaction kettle, then cool down to 10°C, add 265kg of 45% sulfuric acid aqueous solution, control the system temperature not more than 20°C, continue to cool down to 5°C after adding, and then transfer to 3M 3 Stainless steel reaction kettle, add 150kg of catechol methylene ether, start stirring, control the reaction temperature at about 5°C, continue to react for 3-4 hours after adding catechol methylene ether, then add 500kg of water to terminate the reaction; add water and continue Stir for 15min, then put the material into the 5m 3 Enamel reaction kettle, static phase separation, water phase separation, solid phase pumped into 5m 3 Add 100kg of 27% hydrochloric acid, 600kg of benzene, 20kg of catalyst zinc chloride, and start adding hydrogen peroxide (220kg) dropwise when the temperature rises to 65°C. Stop stirring, let stand to separate the phases, separate the water phase to recover the catalyst...
Embodiment 3
[0041] 3m 3 Add 245kg of 50% glyoxylic acid aqueous solution to the enamel reaction kettle, then cool down to 10°C, add 265kg of 45% sulfuric acid aqueous solution, control the system temperature not more than 20°C, continue to cool down to 5°C after adding, and then transfer to 3M 3 Stainless steel reaction kettle, add 150kg of catechol methylene ether, start stirring, control the reaction temperature at about 5°C, continue to react for 3-4 hours after adding catechol methylene ether, then add 500kg of water to terminate the reaction; add water and continue Stir for 15min, then put the material into the 5m 3 Enamel reaction kettle, static phase separation, water phase separation, solid phase pumped into 5m 3 Add 100kg of 27% hydrochloric acid, 600kg of toluene, and 20kg of catalyst ferric chloride to the enamel reaction kettle, and start adding hydrogen peroxide (210kg) dropwise when the temperature rises to 65°C. Stop stirring, let stand to separate the phases, separate th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com