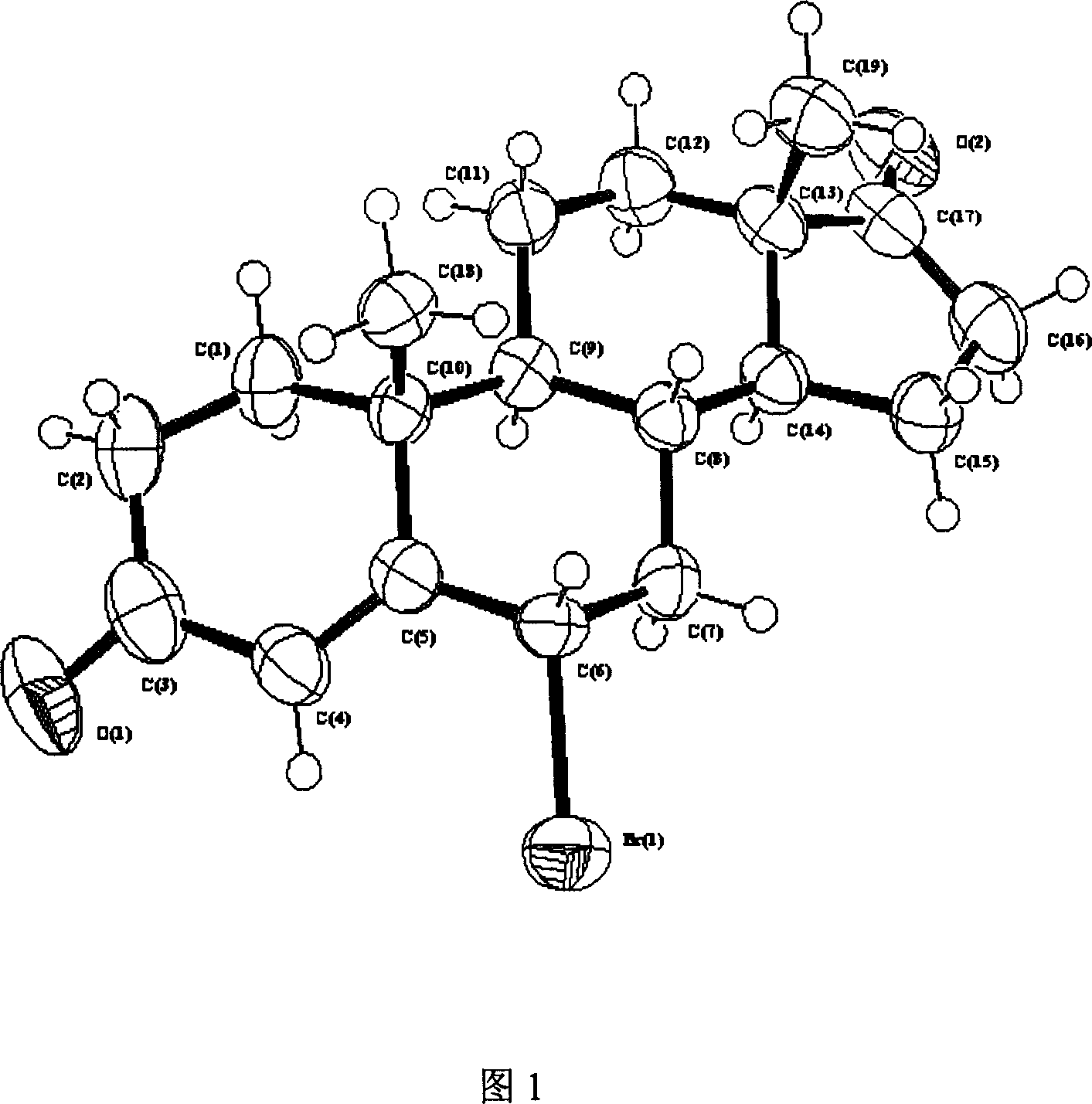
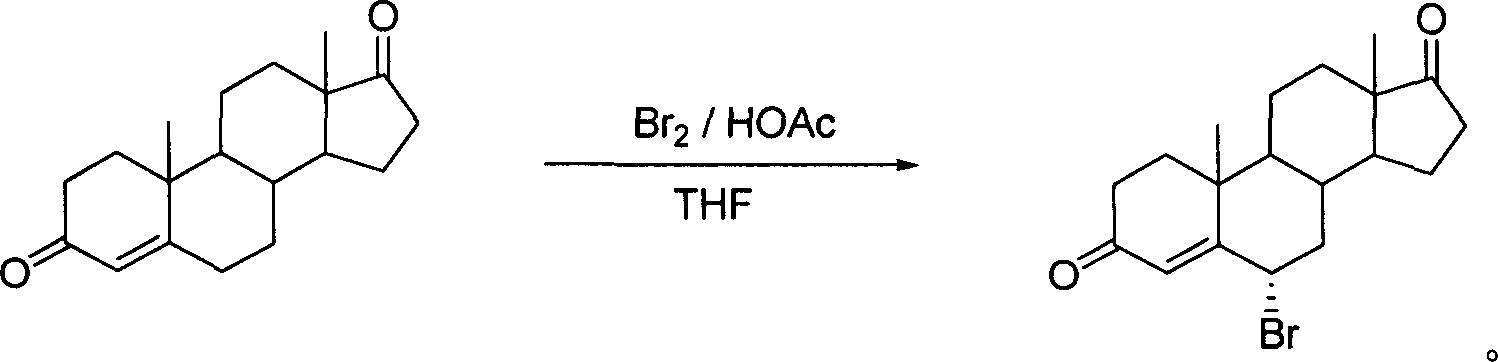6 alpha-bromo- androstane-4-ene-3,17-dione high efficient synthesis method
A synthesis method and technology of synthesis method, which are applied in steroids, organic chemistry and other directions, can solve the problems of inability to effectively separate mixtures, difficult to effectively separate, etc., and achieve the effects of cheap and easy availability of reagents, high yield and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
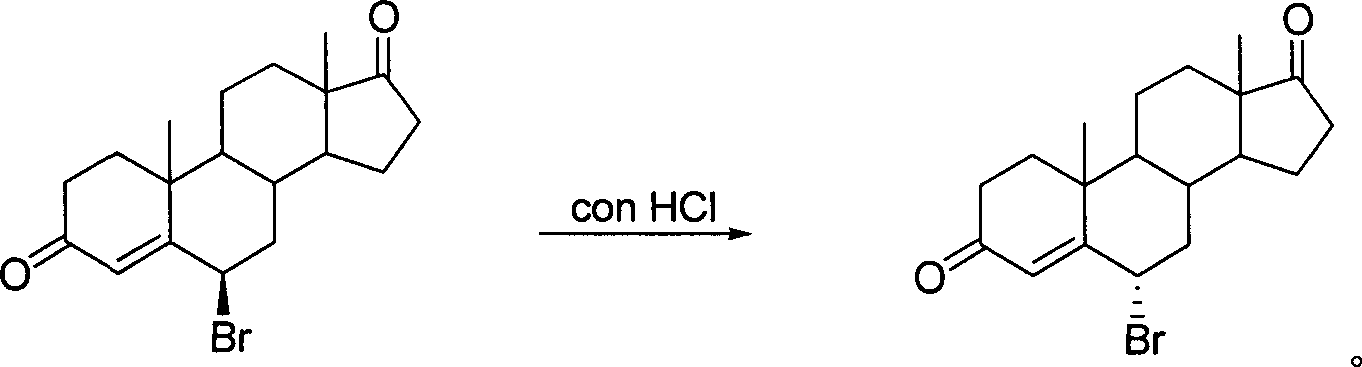
[0016] Preparation of 6α-bromo-androst-4-ene-3,17-dione
[0017] Configuration translocation method: strong acid as reagent and water as reaction medium
[0018] Add 120 mg of 6β-bromo-androst-4-ene-3,17-dione into 4 mL of concentrated hydrochloric acid, stir at room temperature for 21 hours, add 20 mL of ice water, filter, wash the cake with water until neutral, and dry at room temperature to obtain white 100 mg of powdery solid 6α-bromo-androst-4-ene-3,17-dione, yield 83.33%. Crude 1 H-NMR showed that the 6α-bromo product configuration had been purified. Recrystallized with ethyl acetate to obtain fine product [α] 22 D =+143°(CHCl 3 ), mp: 171-172°C. Ms (m / z, %): 364 (M + , 6.79), 285 (M + -79,100); 1 HNMRδ (400HMz, CDCl 3 ): 0.92 (3H, s, 18-CH 3 ), 1.25 (3H, s, 19-CH 3 ), 4.91 (1H, m, 6-H), 6.45 (1H, s, 4-H).
Embodiment 2
[0020] Preparation of 6α-bromo-androst-4-ene-3,17-dione
[0021] Direct synthesis method: Br 2 / HOAc / THF is the reaction system
[0022] Androst-4-ene-3,17-dione 4.0g, 13.97mmol was dissolved in THF40mL, HOAc 4mL was added, bromine 2.33g, 13.97mmol was added dropwise at room temperature, the dropwise was completed in 15 minutes, and the reaction was basically completed after stirring for 15 minutes. Evaporate THF under reduced pressure at a bath temperature of 40-45°C, analyze with 80 mL of ice water, filter, wash the filter cake with water until it is neutral, and dry it by infrared to obtain the crude yellow solid product 6α-bromo-androst-4-ene- 3,17-diketone 4.96g, yield: 97.2%: mp: 164-165°C turns black and decomposes, crude product determination 1 H-NMR showed that the configuration of the 6α-bromo product was pure. Ethyl acetate recrystallized to obtain fine product[α] 22 D =143°(CHCl 3), mp: 171-172°C. Ms (m / z, %): 364 (M + , 6.79), 285 (M + -79,100); 1 H NMRδ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com