Method for synthesizing 21-hydroxy-17-(1-oxopropoxy)pregna-4-ene-3,20-dione
A technology of oxypropoxyl and hydroxypropionyl, applied in the production of steroids, bulk chemicals, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
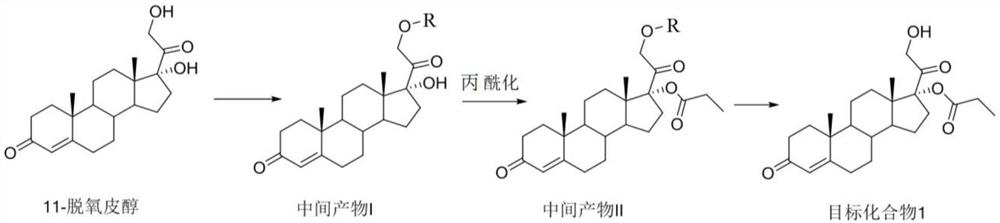
[0066] Preparation of Intermediate I
[0067]
[0068] where R = DMTr
[0069] Compound 11-deoxycortisol (1.04g, 3.0mmol, 1eq.) was dissolved in 10mL of anhydrous pyridine, and dried DMTrCl (1.2-1.5eq) was dissolved in 5mL of anhydrous dichloromethane, and reacted at room temperature Add the dichloromethane solution of DMTrCl dropwise in the product solution, and react at room temperature for 4 hours; quench the reaction with methanol, evaporate the solvent to dryness in an oil pump, and obtain intermediate product I with a yield of 85% (the next step reaction can be directly carried out without aftertreatment, the following One-step solvent environment and catalyst are similar to this step reaction).
[0070] 1 HNMR (600MHz, CDCl 3 )δ(ppm)7.25-7.31(m,5H,H-DMTr),7.15-7.18(m,4H,H-DMTr),6.81-6.84(m,4H,H-DMTr),5.73(1H,s, H-4),4.65(1H,dd,J=19.8,4.8Hz,H-21),4.30(1H,dd,J=19.8,4.8Hz,H-21),3.80(6H,s),2.71( s,1H,17-OH),2.66-2.71(m,1H,H-16β),2.27-2.45(m,4H),1.19(3H,s,H-19),0.96-...
Embodiment 2
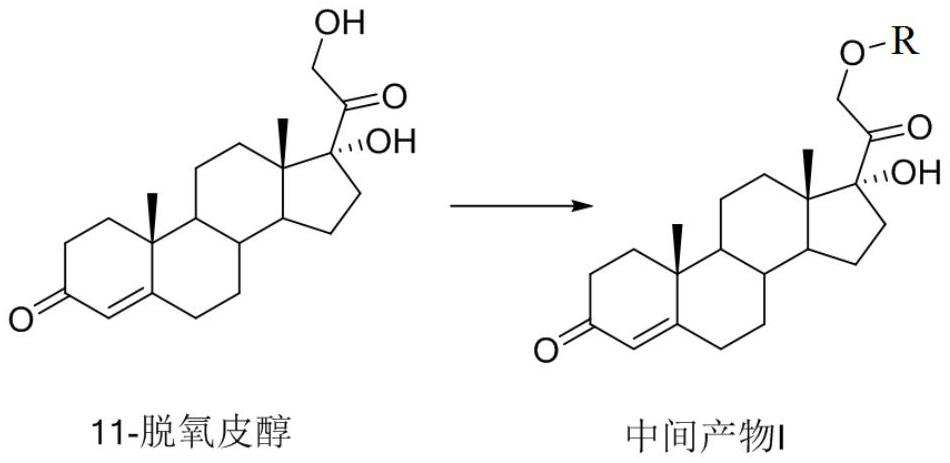
[0074] Preparation of Intermediate II
[0075]
[0076] where R = DMTr
[0077] Under nitrogen protection, the intermediate product I (1eq.) was dissolved in 5mL of anhydrous dichloromethane, DMAP (0.1eq.) was added to the above solution, triethylamine (1.2eq.) and propionic anhydride or propionyl chloride were added dropwise (1.2eq.), after dropping, react at 40°C for 12 hours, and evaporate the solvent to obtain the intermediate product II.
[0078] Or under nitrogen protection, the intermediate product I (1eq.) was dissolved in 5mL of anhydrous pyridine, DMAP (0.1eq.) was added to the above solution, triethylamine (1.2eq.) and propionic anhydride or propionyl chloride were added dropwise (1.2eq.), after dropping, react at 80°C for 4 hours, and evaporate the solvent to obtain the intermediate product II. (This step reaction can be processed without strict purification and evaporated to dryness to directly carry out the next step to remove the DMTr protecting group to ob...
Embodiment 3
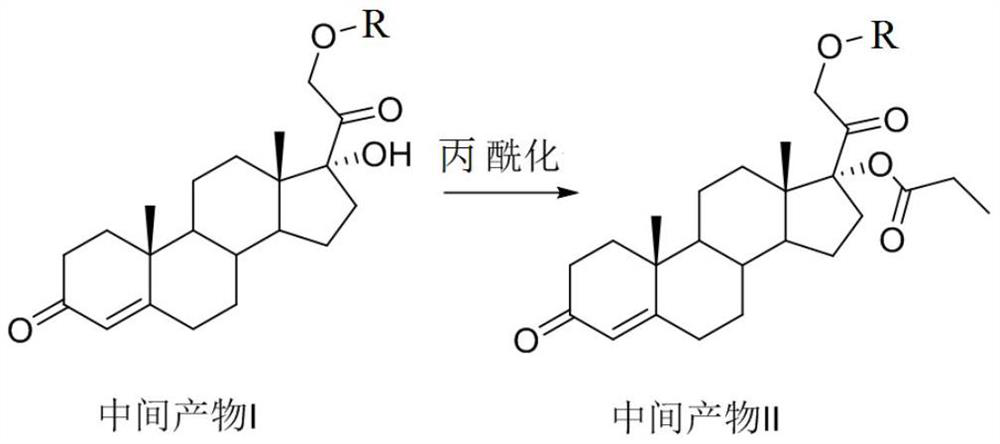
[0082] Preparation of target compound 1 (21-hydroxy-17-(1-oxopropoxy)pregna-4-ene-3,20-dione)
[0083]
[0084] The concentrated intermediate product II was dissolved in ethyl acetate solution, slowly added dropwise with 0.5M hydrochloric acid solution or 2% trifluoroacetic acid-ethyl acetate solution at 0°C, and reacted at 0°C for 5 minutes to remove DMTr Protecting group, at 0°C, add 5% aqueous sodium bicarbonate and stir to neutralize the acid in the reaction system. The ethyl acetate organic layer is washed twice with 5% aqueous sodium bicarbonate to remove the organic layer of ethyl acetate. Acid and other water-soluble impurities, the organic layer of ethyl acetate was dried over anhydrous sodium sulfate, part of the ethyl acetate solvent was evaporated, and petroleum ether was added to the remaining small amount of ethyl acetate dissolved, and ethyl acetate-petroleum ether (5:1) Recrystallization was carried out in a system with 10 times the amount of solvent to obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com