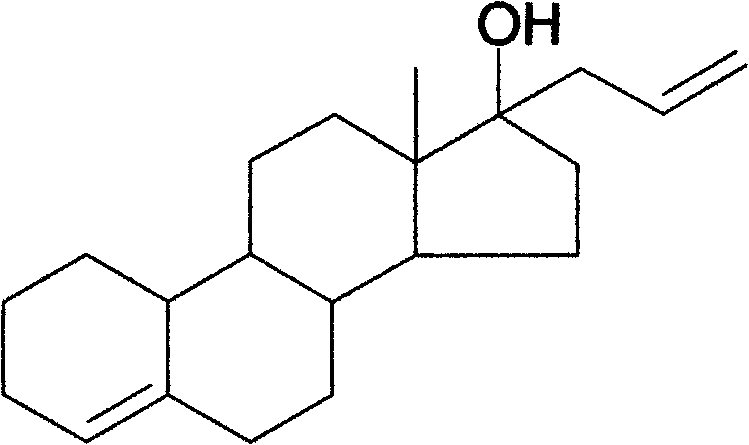
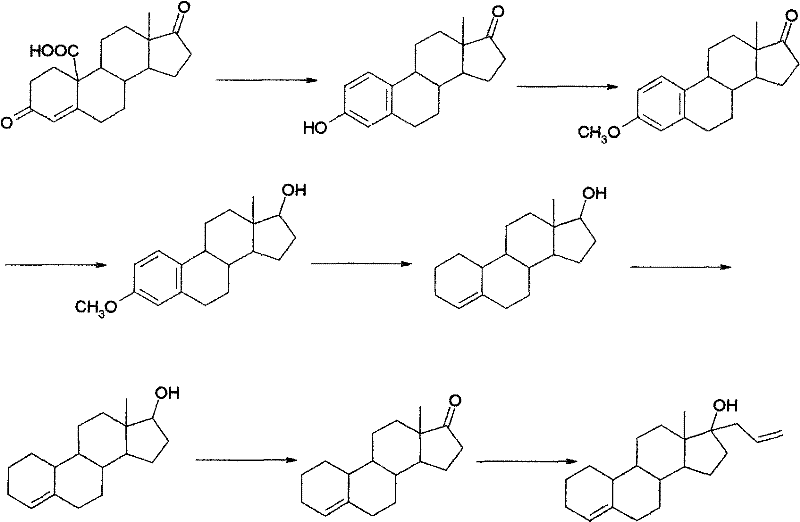
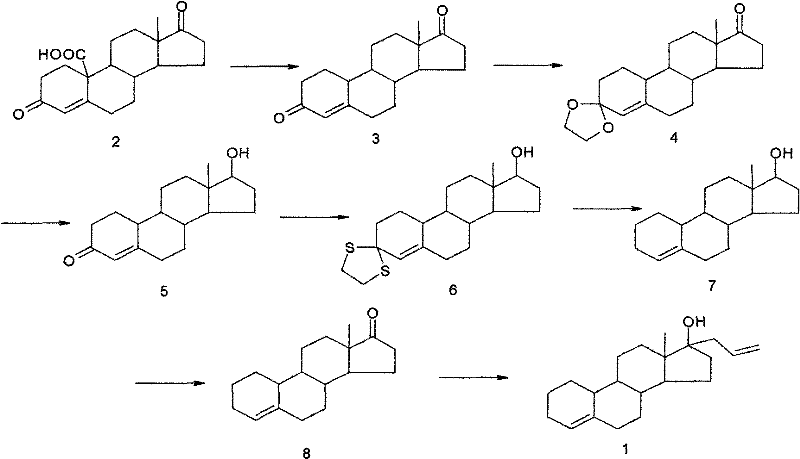
Preparation method of allylestrenol
A technology of allylestradiol and estradiol, which is applied in the field of preparation of the compound allylestradiol, can solve the problems of low yield, high cost, difficult purification and the like of intermediate 5, achieves improved quality and yield, and is suitable for technological production. , the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0040] (1) Preparation of compound ③ from estro-4-ene-3,17-dione
[0041] Add glacial acetic acid (75ml) into the reaction vessel, start stirring, put in compound ② (10g, 36.7mmol) and ethanedithiol (4.0ml, 47.6mmol), adjust the temperature to 5-10°C, add dropwise 4.5ml trifluoro Boronium diethyl ether was added dropwise for about 15 minutes, and then the reaction was kept at 5-10°C for 2.5 hours. Then slowly pour the reaction solution into an aqueous solution made of 60g NaOH dissolved in 450ml water at about -5°C, stir for about 30 minutes after the water analysis is completed, let it stand for about 1 hour, filter with suction, wash the filter cake with water until it is neutral, and drain it. , recrystallized to obtain white solid compound ③ (12.7g, 36.4mmol) with a yield of 99%, m.p=220-223°C.
[0042] (2) Preparation of compound ④
[0043] Add ethylene glycol (40ml) and triethyl orthoformate (40ml) into the reaction vessel, start stirring, put in compound ③ (10g, 28.7m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com