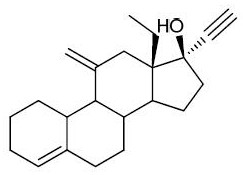
Preparation method of desogestrel
A desogestrel, monohydrate technology, applied in the production of steroids, organic chemistry, bulk chemicals, etc. The effect of high yield, high stability and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
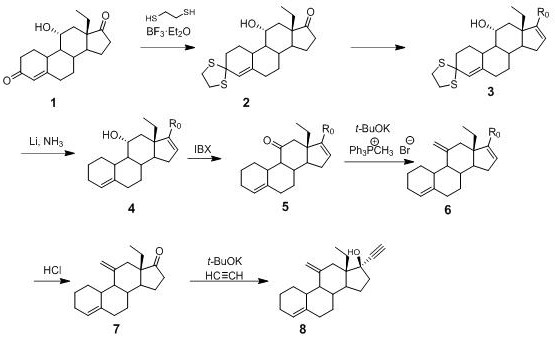
[0033] Synthesis of compound 2: In the reactor, 1.5kg of 11α-hydroxy-18-methyl-estr-4-ene-3,17-dione (compound 1), 7.5kg of glacial acetic acid and p-toluenesulfonic acid monohydrate were successively 150g of PTSA (PTSA), under full stirring, 560g of ethanedithiol was added dropwise at 20~30°C for about 30 minutes; after the dropwise addition, the temperature was kept for 3 hours; the reaction solution was slowly poured into -5°C sodium hydroxide aqueous solution ( 7.5kg of sodium hydroxide and 30kg of water), separated and stirred for 2 hours, centrifuged, and the filter cake was rinsed with water; recrystallized from methanol to obtain 1.7kg of white solid (compound 2), mass yield 113%, HPLC purity: 98 %.
[0034] Synthesis of compound 3a: In the reaction kettle, add toluene (6.0kg), compound 2 (1.0kg), piperidine (600g) and PTSA (100g) successively, and reflux for 4 hours; cool to room temperature, suction filtration; in the filtrate Silica gel (500 g) was adde...
Embodiment 2
[0040]
[0041] Synthesis of Compound 2: In Reference Example 1, 50 g of (Compound 1) was added to obtain 58 g of (Compound 2); the mass yield was 116%, and the HPLC purity: 98%.
[0042] Synthesis of compound 3b: In the reaction kettle, add toluene (300g), compound 2 (50g), pyrrolidine (30g) and PTSA (5g) in turn, and reflux for 4 hours; cool to room temperature, suction filtration; add silica gel to the filtrate (25g), stirred for 1 hour, and suction filtered; the filtrate was concentrated under reduced pressure to an oily substance, which was recrystallized from ethyl acetate-petroleum ether to obtain compound 3b (45g), mass yield 90%, HPLC purity: 97%.
[0043] Synthesis of compound 4b: add liquid ammonia (400mL) in the low temperature reaction kettle, cool to -45°C~-50°C, add metallic lithium (6g) in 3 batches, add and stir for 1 hour, dropwise add the tetrahydrofuran solution of compound 3b (40 g of compound 3b was dissolved in 320 mL of tetrahydrofuran), about 1 hour...
Embodiment 3
[0048]
[0049] Synthesis of Compound 2: In Reference Example 1, 50 g of (Compound 1) was added to obtain 58 g of (Compound 2); the mass yield was 116%, and the HPLC purity: 98%.
[0050] Synthesis of compound 3c: In the autoclave, add compound 2 (50g), diethylamine (300g) and PTSA (5g) in turn, react at 120°C for 8 hours; cool to room temperature, release the pressure, open the autoclave, suction filtration, and concentrate , add toluene to dissolve, add silica gel (25g), stir for 1 hour, suction filtration; the filtrate is concentrated under reduced pressure to an oily substance, recrystallized from ethyl acetate-petroleum ether to obtain compound 3c (15g), mass yield 30%, HPLC purity: 92 %.
[0051] Synthesis of compound 4c: add liquid ammonia (100 mL) in the low temperature reaction kettle, cool to -45°C~-50°C, add metallic lithium (1.5g) in 3 batches, add and stir for 1 hour, dropwise add the tetrahydrofuran of compound 3b The solution (10 g of compound 3b was dissolv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com