Method utilizing cyclodextrin to assist plant sterol composition to prepare androstane-4-ene-3,17-diketone
A technology for phytosterol and composition, applied in the field of preparing androst-4-ene-3, can solve the problems of low solubility, increase of miscellaneous bacteria, excessive fermentation of microorganisms and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1-a
[0061] Species: Mycobacterium: Mycobacterium fortuitum. NRRL B-8153.
[0062] Take 10 different batches of corn steep liquor and ferment them respectively according to the quantity and process in this example. Except corn steep liquor, other materials are the same batch of materials.
[0063] Seed culture stage:
[0064] Seed medium (per liter):
[0065] 40ml corn syrup
[0066] 5.2g NaNO 3 ,
[0067] 0.8g NH 4 h 2 PO 4
[0068] 5.3g glucose
[0069] The rest is sterile water
[0070] Medium volume: 200ml / flask
[0071] Inoculum volume: Inoculate 3ml of cell suspension from agar slant per 200ml of new medium
[0072] Container: 1000ml conical flask
[0073] Stirring: Shaker (1”throw) 200rpm
[0074] Temperature: 30°C
[0075] Growth time: 72 hours culture
[0076] Note: A good culture organism is indicated if a yellow cell pellet forms at the bottom
[0077] method:
[0078] Inoculum for biotransformation was prepared in culture medium. Inoculate the Erlenmeye...
Embodiment 1-1-b
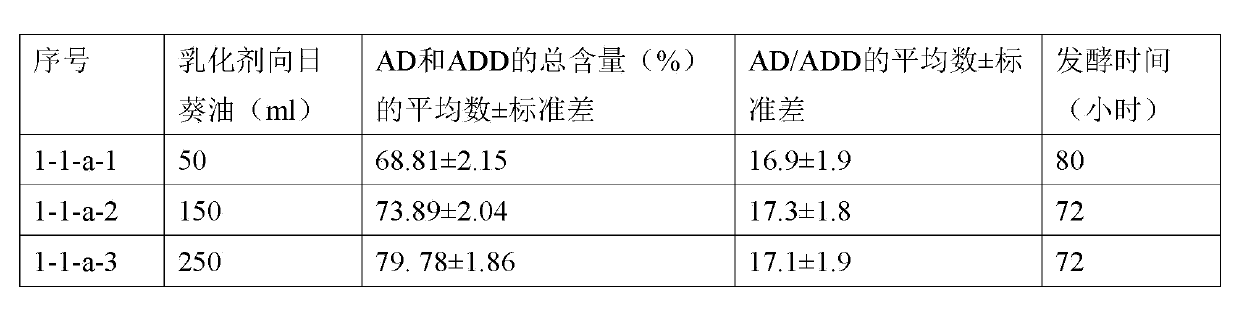
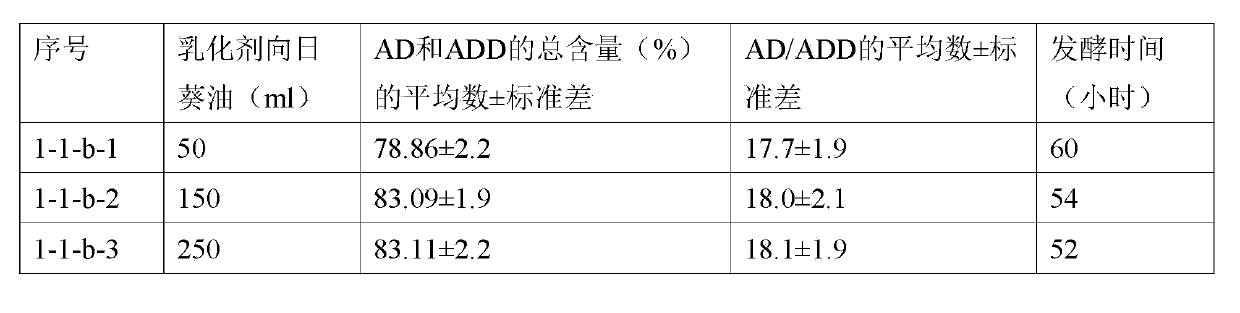
[0099] The material and method of this example are basically the same as those of Example 1-1-a, except that 0.01 mole of γ-cyclodextrin is added to the sunflower oil in which the phytosterol composition (0.05 mole) is dispersed. See the table below for the fermentation time of steroidal fermentation culture.
[0100]
Embodiment 1-2-b
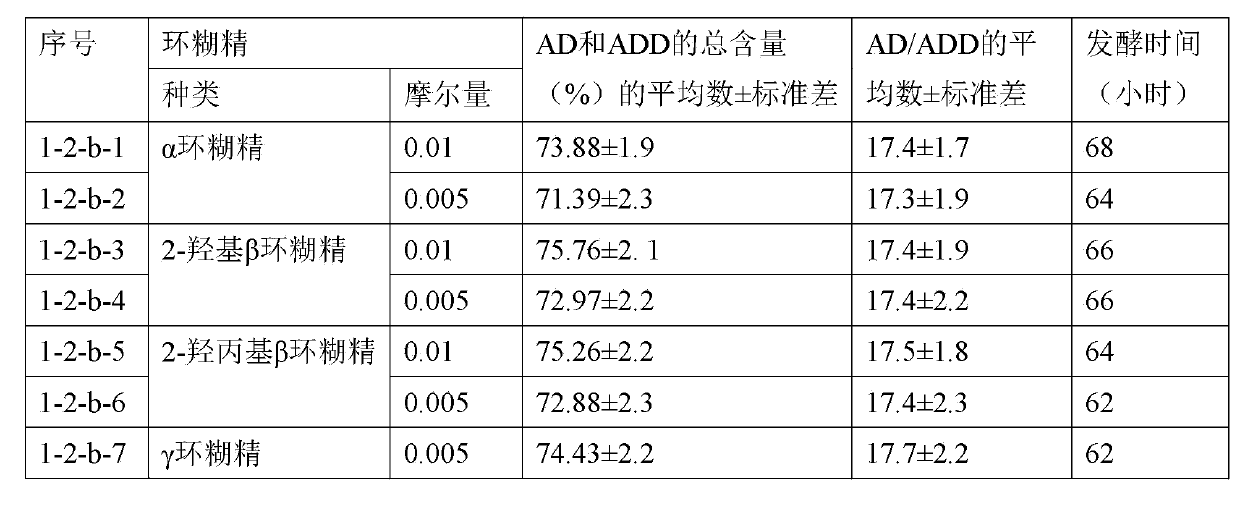
[0102] The materials and methods of this example and Example 1-1-b are basically the same, the difference is that the amount of emulsifier sunflower oil is 50ml, and the type, molar amount and results of cyclodextrin are shown in the table below.
[0103]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com