Preparation method and application of glycopyrronium bromide chiral antipode
A technology of glycopyrronium bromide and enantiomers, which is applied in the field of medicine and can solve problems such as sales increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Illustrate with respect to summary of the invention below, summary of the invention includes cited example but not limited to following example:
[0054] Preparation of (S)-N-benzyl-3-hydroxysuccinimide
[0055] 33.50 g of L-malic acid was suspended in 300 mL of toluene, 26.80 g of benzylamine was added dropwise, and heated to reflux for reaction. After the reaction, the toluene was evaporated under reduced pressure, and 40.82 g of white crystals were obtained by recrystallization with 50 mL of dichloromethane (yield 79 %), mp 104~106 ℃; MS (m / z): 228 (M+H + ); 1 H NMR (CD 3 OD) δ: 2.48 (dd, 1H), 3.05 (dd, 1H), 6.13 (d, 1H), 7.25 (d, 3H), 7.32 (t, 2H).
[0056] Preparation of (S)-N-benzylpyrrolidin-3-ol
[0057] Suspend 7.10 g of lithium aluminum hydride in 250 mL of anhydrous tetrahydrofuran, add dropwise a solution of 15.00 g of (S)-N-benzyl-3-hydroxysuccinimide in 150 mL of anhydrous tetrahydrofuran, and heat to reflux after the addition is complete. After the r...
Embodiment 2
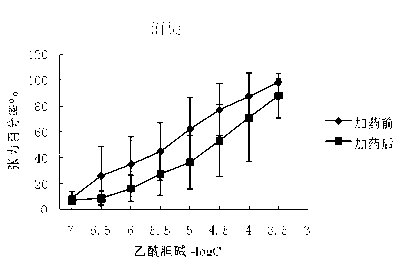
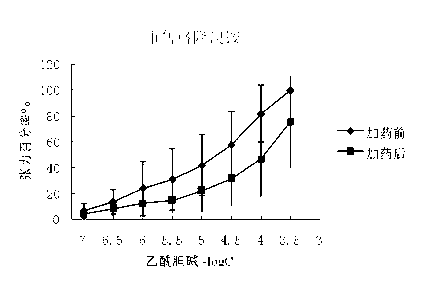
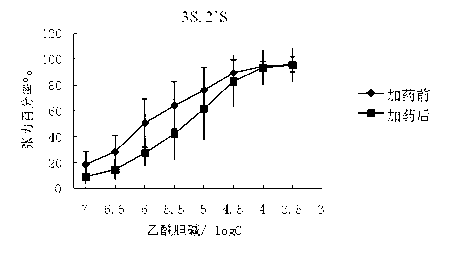
[0089] The M receptor agonist acetylcholine (Ach) stimulates the M choline receptors on the smooth muscle to produce a smooth muscle contraction effect. In this study, the cecum and trachea of guinea pigs were selected as objects to study the competitive antagonism of glycopyrronium bromide chiral enantiomers in vitro against acetylcholine ( Ach) agonistic effect on smooth muscle muscarinic receptors, and commercially available glycopyrronium bromide and racemic glycopyrrolate bromide were used as control drugs. Commercially available glycopyrronium bromide is a mixture of (3R, 2′S) and (3S, 2′R), and the racemic drugs are (3S, 2′S), (3R, 2′S), (3R, 2′R ) and (3S, 2′R) mixtures.
[0090] In this study, the above muscarinic receptor antagonistic drug screening system was used to initially screen out the chiral enantiomer of glycopyrronium bromide with the strongest cholinergic antagonistic effect. The specific implementation plan is as follows:
[0091] (1). Anticholinergic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com