Preparation method of anti-new crown drug Paxlovid intermediate
A technology for intermediates and drugs, applied in the field of preparation of intermediates of anti-new crown drug Paxlovid, can solve the problems of cumbersome synthesis steps, high comprehensive cost, large amount of three wastes, etc., and achieves high operability, low cost, and reduced amount of three wastes. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
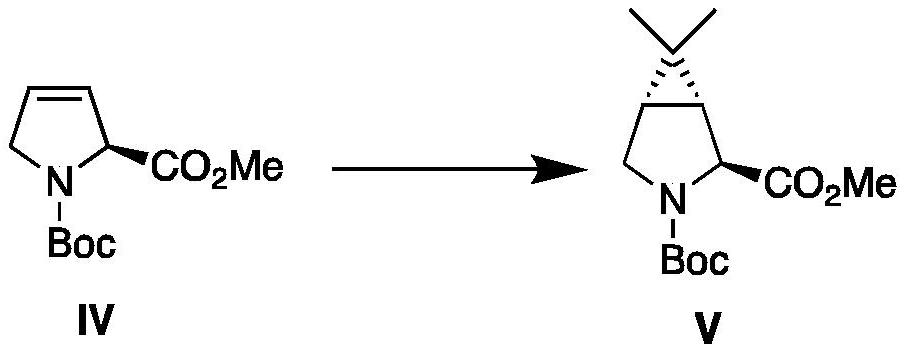
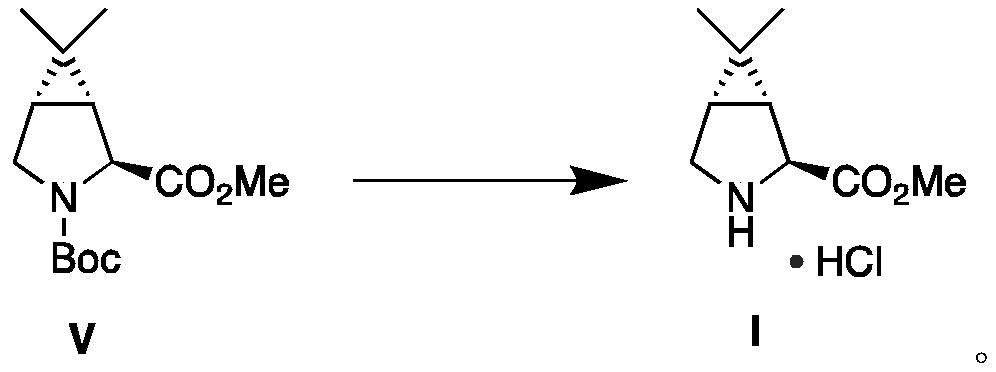
Examples
Embodiment 1
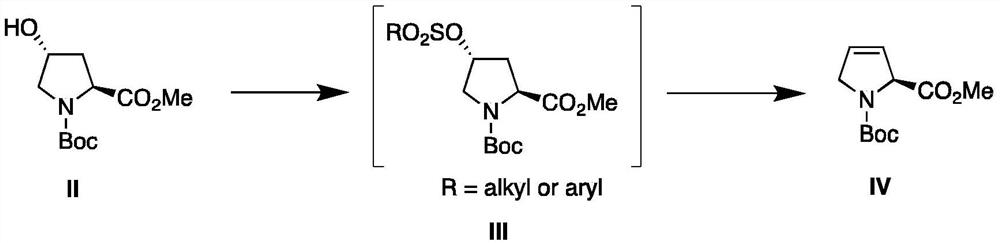
[0038] Preparation of Compound IV
[0039] Add 981g of compound II, 450g of triethylamine and 3L of dichloromethane into a 5L round bottom flask, add 801g of p-toluenesulfonyl chloride under ice cooling, stir until compound II disappears, add water to quench, extract and separate the liquids, and the water phase is again washed with 2L dichloromethane Chloromethane extraction once. The combined organic phases were dried over anhydrous magnesium sulfate and concentrated to dryness under reduced pressure.
[0040] The obtained p-toluenesulfonate intermediate was dissolved in 4L of dioxane solution, 672g of potassium tert-butoxide was added under ice-cooling, and the temperature was raised to reflux until the intermediate disappeared. 1 L of 10% ammonium chloride aqueous solution was added to quench, dioxane was removed under reduced pressure, and the aqueous phase was extracted twice with 2 L of ethyl acetate. The combined organic phases were dried over anhydrous magnesium sul...
Embodiment 2
[0045] Preparation of Compound IV
[0046] Add 981g of compound II, 450g of triethylamine and 3L of dichloromethane into a 5L round-bottomed flask, add 930g of p-nitrobenzenesulfonyl chloride under ice cooling, stir until compound II disappears, add water to quench, extract and separate, and use the water phase again 2L dichloromethane extracted once. The combined organic phases were dried over anhydrous magnesium sulfate and concentrated to dryness under reduced pressure.
[0047]The obtained p-nitrobenzenesulfonate intermediate was dissolved in 4L of dioxane solution, 576g of sodium tert-butoxide was added under ice-cooling, and the temperature was raised to reflux until the intermediate disappeared. 1 L of 10% ammonium chloride aqueous solution was added to quench, dioxane was removed under reduced pressure, and the aqueous phase was extracted twice with 2 L of ethyl acetate. The combined organic phases were dried over anhydrous magnesium sulfate, and concentrated to dryn...
Embodiment 3
[0049] Preparation of Compound IV
[0050] Add 981g of compound II, 450g of triethylamine and 3L of dichloromethane into a 5L round-bottomed flask, add 481g of methanesulfonyl chloride under ice bath, stir until compound II disappears, add water to quench, extract and separate the liquids, and use 2L of dichloromethane again for the water phase methane extraction once. The combined organic phases were dried over anhydrous magnesium sulfate and concentrated to dryness under reduced pressure.
[0051] The obtained mesylate intermediate was dissolved in 4L of toluene solution, 1220g 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) was added under ice cooling, and the temperature was raised to reflux to the intermediate disappear. Add 1 L of 10% hydrochloric acid aqueous solution to quench, separate liquid extraction, and extract the aqueous phase with 2 L of toluene once. The combined organic phases were dried over anhydrous magnesium sulfate, and concentrated to dryness under reduced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com