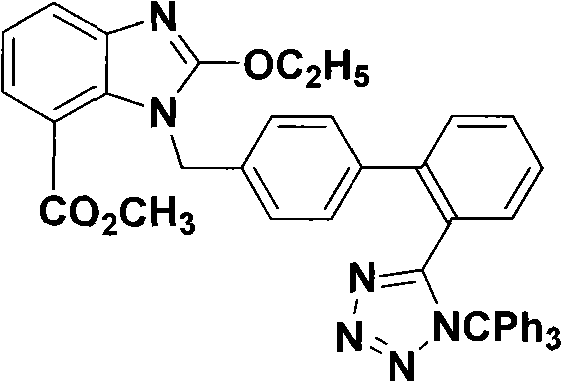
Novel preparation of trityl group candesartan cilexetil intermediate
A technology of trityl candesartan and a new method is applied in the new field of preparation of trityl candesartan cilexetil intermediates, and can solve the problem that methyl benzoate is not easily available, complicated to operate, and a large amount of candesartan medoxomil. Preparation difficulties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
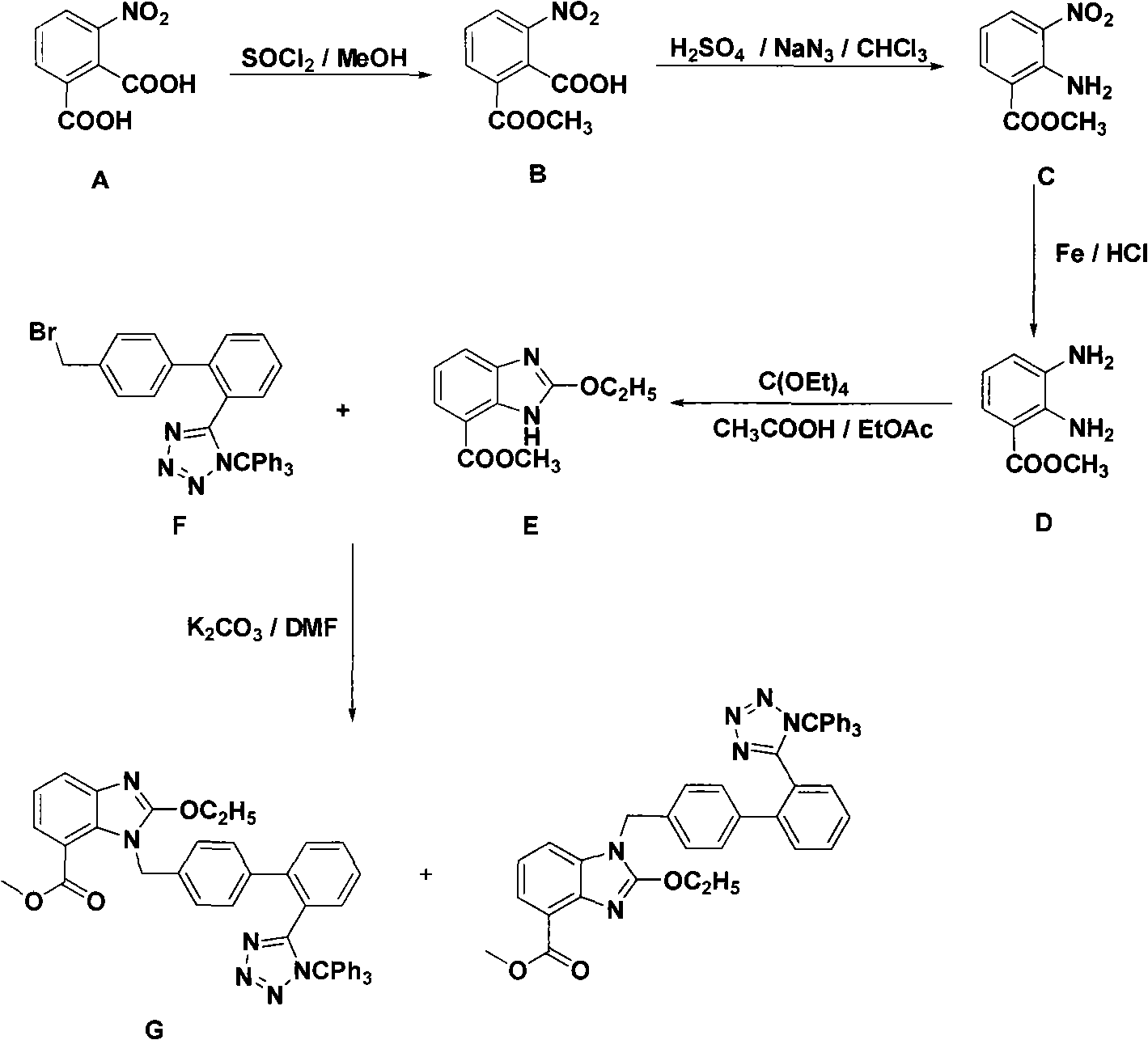
Examples
Embodiment 1
[0010] Example 1 Preparation of 2-methyl formate-6-nitro-benzoic acid
[0011] Add 200g of 3-nitrophthalic acid and 1L of methanol into the reaction flask. 104 mL of thionyl chloride was added dropwise in an ice-water bath, and the drop was completed in 2 hours; the temperature was raised to reflux, and the reaction was carried out for 24 hours. The reaction mixture was concentrated to dryness, washed with petroleum ether, and filtered to obtain 210.5 g of 2-methyl formate-6-nitro-benzoic acid as a white solid, with a yield of 99%.
Embodiment 2
[0012] Example 2 Preparation of 2-amino-3-nitro-benzoic acid methyl ester
[0013] Add 100g of 2-methylformate-6-nitro-benzoic acid, 800mL of chloroform, and 133.3mL of 98% sulfuric acid into the reaction flask in sequence. At room temperature, 56 g of sodium azide was added in batches, and the addition was completed in about 100 minutes; the temperature was raised to 50° C., and the reaction was carried out for 22 hours. The reaction mixture was cooled to room temperature, decanted off the solvent, washed with chloroform, added 400 mL of water, and filtered to obtain 78.4 g of a yellow solid with a yield of 90%.
Embodiment 3
[0014] Example 3 One of the preparation methods of 2-ethoxy-4-formic acid methyl ester-3-hydrogen-benzimidazole
[0015] 5g 2-amino-3-nitro-benzoic acid methyl ester, 50mL concentrated hydrochloric acid, 25g SnCl 2 2H 2 O was added to the reaction flask in turn, and reacted at room temperature for 5 hours. The pH of the reaction mixture was adjusted to 8 with saturated sodium carbonate solution, extracted with ethyl acetate, the organic phases were combined, and the solvent was distilled off to obtain 4 g of light yellow solid of methyl 2,3-diamino-benzoate.
[0016] The 2,3-diamino-benzoic acid methyl ester obtained above, 5.6 mL of glacial acetic acid, and 6 g of tetraethyl orthoformate were sequentially added into the reaction flask, and reacted at room temperature for 3 hours. Concentrate the reaction mixture to dryness, add 50mL H 2 O, cooled to 5°C, and filtered to obtain 4.25 g of white solid 2-ethoxy-4-formic acid methyl ester-3-hydrogen-benzimidazole, with a yield ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com