Tosilate of benzodiazepine derivative, its crystal forms, their preparation method and application
A technology of tosidic acid and citric acid, which is applied in the preparation of sulfonate, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of unsatisfactory chemical and optical purity and affecting the stability of compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the synthesis of salt:
[0047] Get formula (I) compound base (according to patent US200,700,934,75A preparation) 1g is dissolved in 6ml ethyl acetate, then 0.39g Toxic acid (equal molar ratio) is dissolved in 1ml methyl alcohol, and is added dropwise to formula ( I) in the ethyl acetate solution of the compound base, stirred and crystallized, suction filtered, and dried under reduced pressure to obtain the toxic acid salt of the compound of formula (I), 0.94 g of white solid, and the yield was 75.0%. HPLC: 99.18%, optical purity: 99.87%.
Embodiment 2
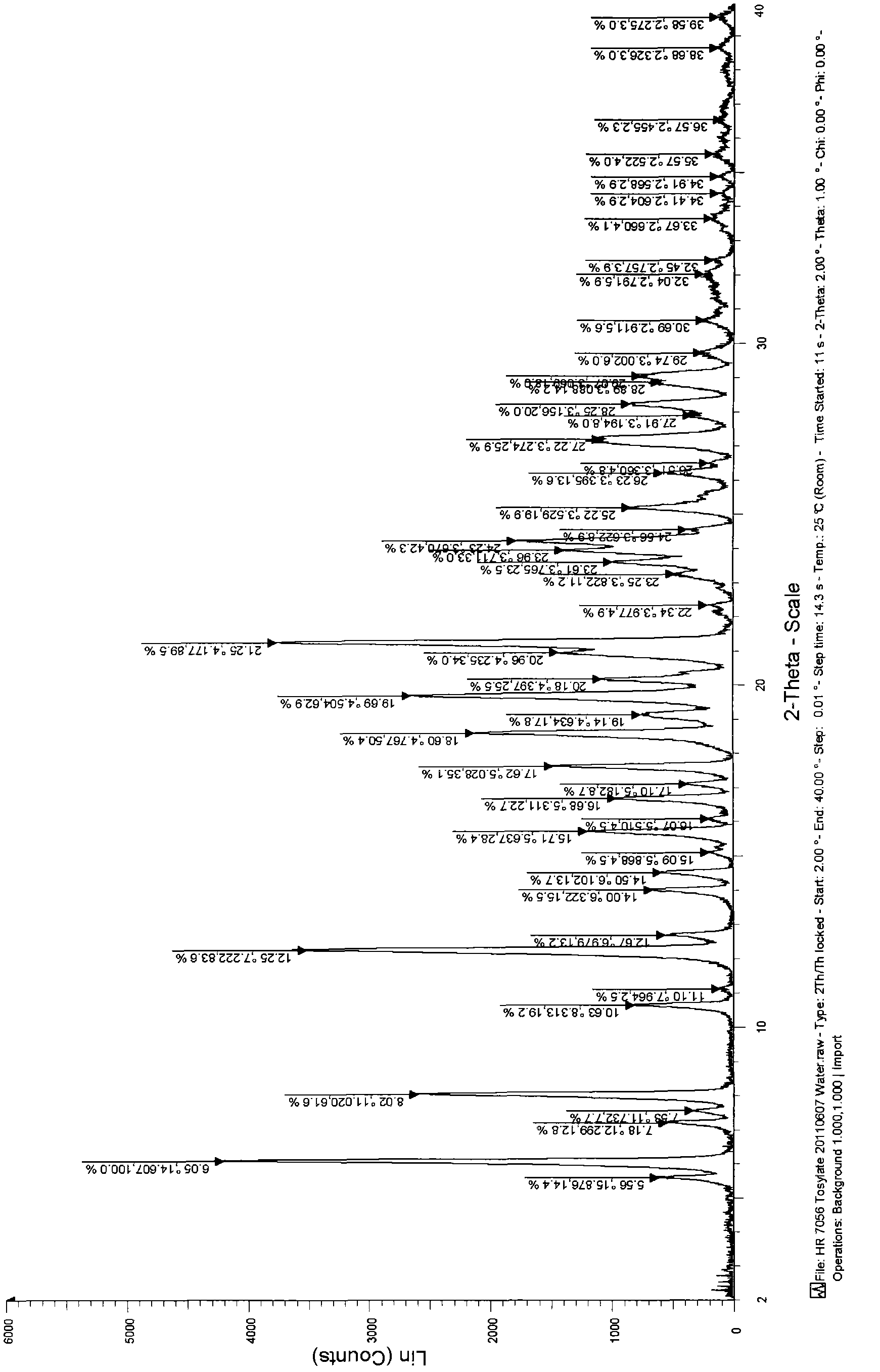
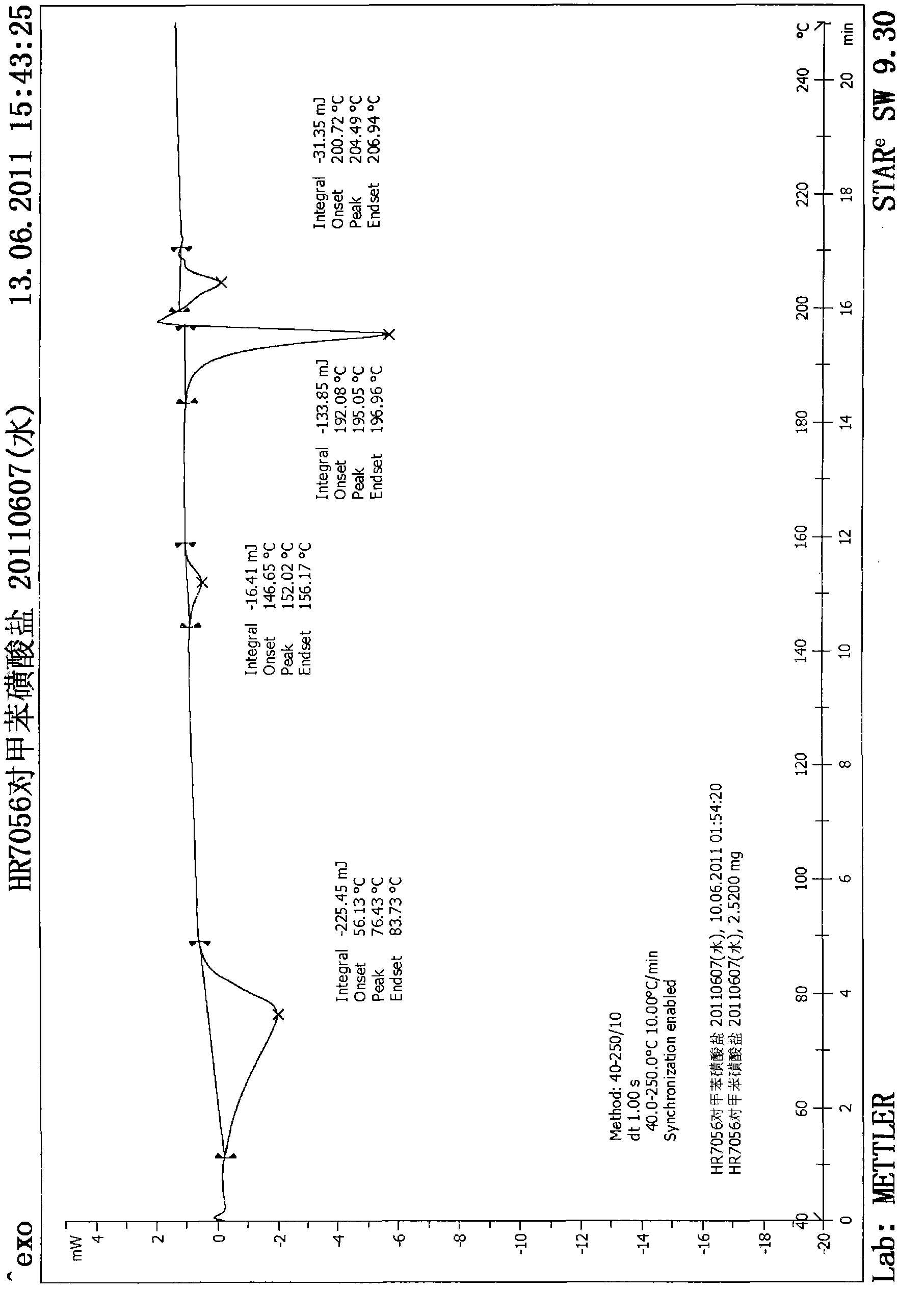
[0049] Add 1.0 g of the toxic acid salt of the compound of formula (I) obtained in Example 1 into a 50 ml round-bottomed flask, add 20 ml of water, and heat to reflux for 10 min to completely dissolve the solid, stop heating, filter while hot, and cool to crystallize , the obtained product was dried under reduced pressure at 45° C. overnight to obtain 0.64 g of a white solid with a yield of 64%. The X-ray diffraction spectrum figure of this crystalline sample is shown in figure 1 . The crystallization at about 6.05 (14.61), 7.18 (12.30), 8.02 (11.02), 12.25 (7.22), 14.00 (6.32), 14.50 (6.10), 15.71 (5.64), 16.68 (5.31), 17.62 (5.03), 18.60 (4.77), 19.69(4.50), 21.25(4.18), 24.23(3.67), 25.22(3.53) and 27.22(3.27) have characteristic peaks, see the DSC spectrum figure 2 , there are characteristic absorption peaks at about 152.02°C, 195.05°C and 204.49°C, defining this crystal form as I crystal form.
Embodiment 3
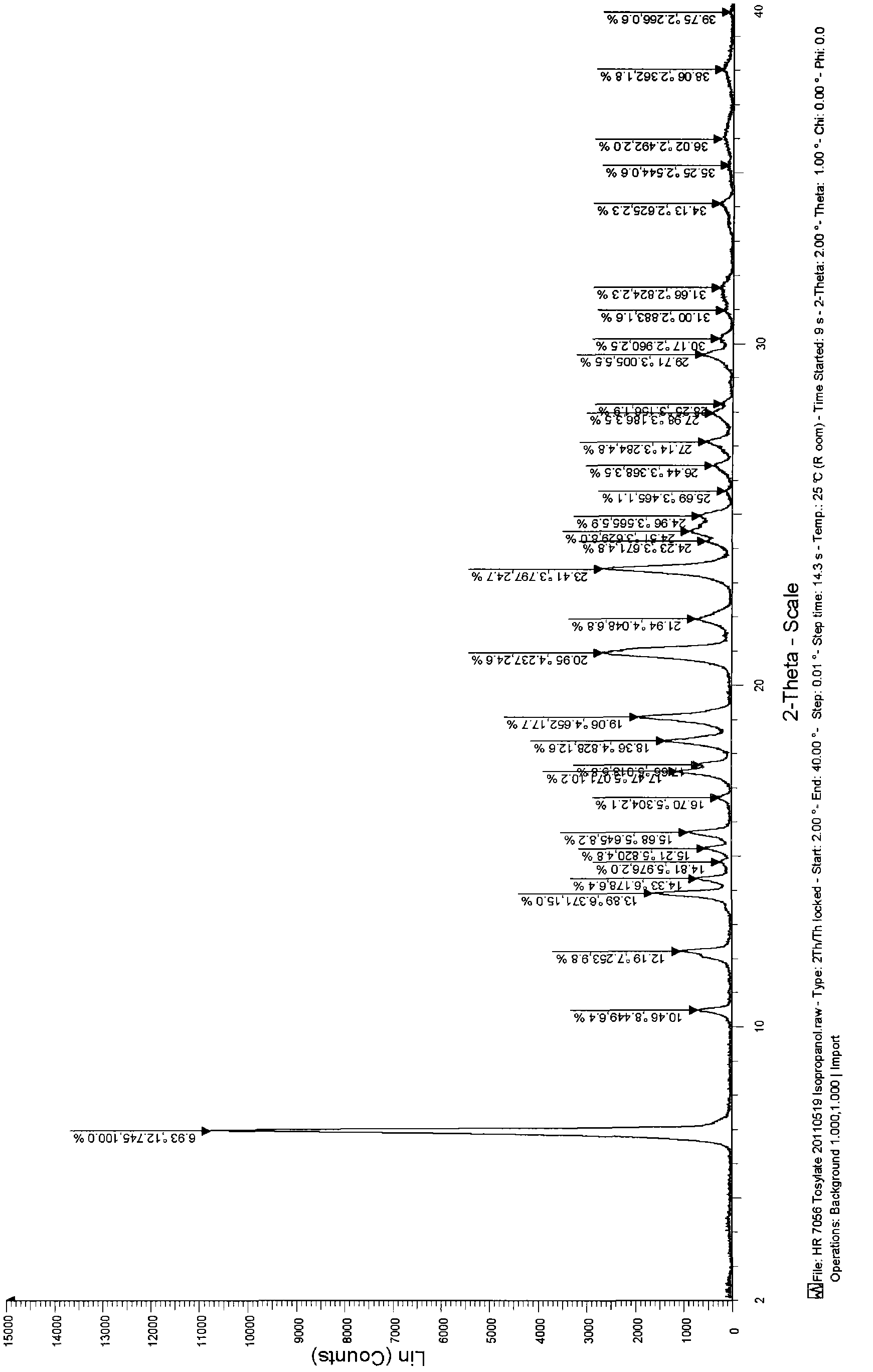
[0051] Toxinate 1.0g of the compound of formula (I) obtained in Example 1 is added in a 10ml round-bottomed flask, added in 5.0ml of 50% isopropanol aqueous solution, heated to reflux to make the solid dissolve completely, stop heating, and cool After crystallization, the obtained product was dried under reduced pressure at 45° C. overnight to obtain 0.72 g of a white solid, with a yield of 72%. The X-ray diffraction and DSC spectrograms of the crystalline sample are studied and compared, and it is determined that the product is the I crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com