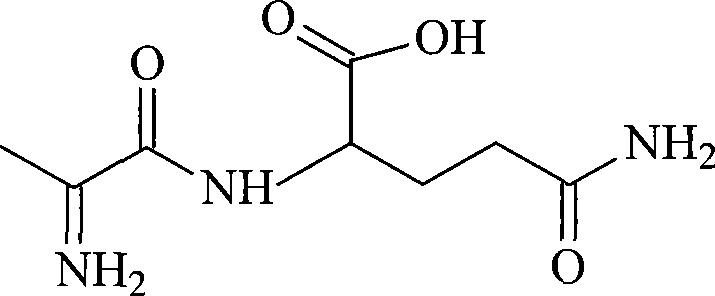
L-alanyl-L-glutamine compound and synthetic method thereof
A technology of glutamine and a synthesis method, applied in the field of medicine, can solve the problems of complex synthesis process, high production cost, low yield harm and the like, and achieve the effects of simple synthesis process, improved yield, and few reaction by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
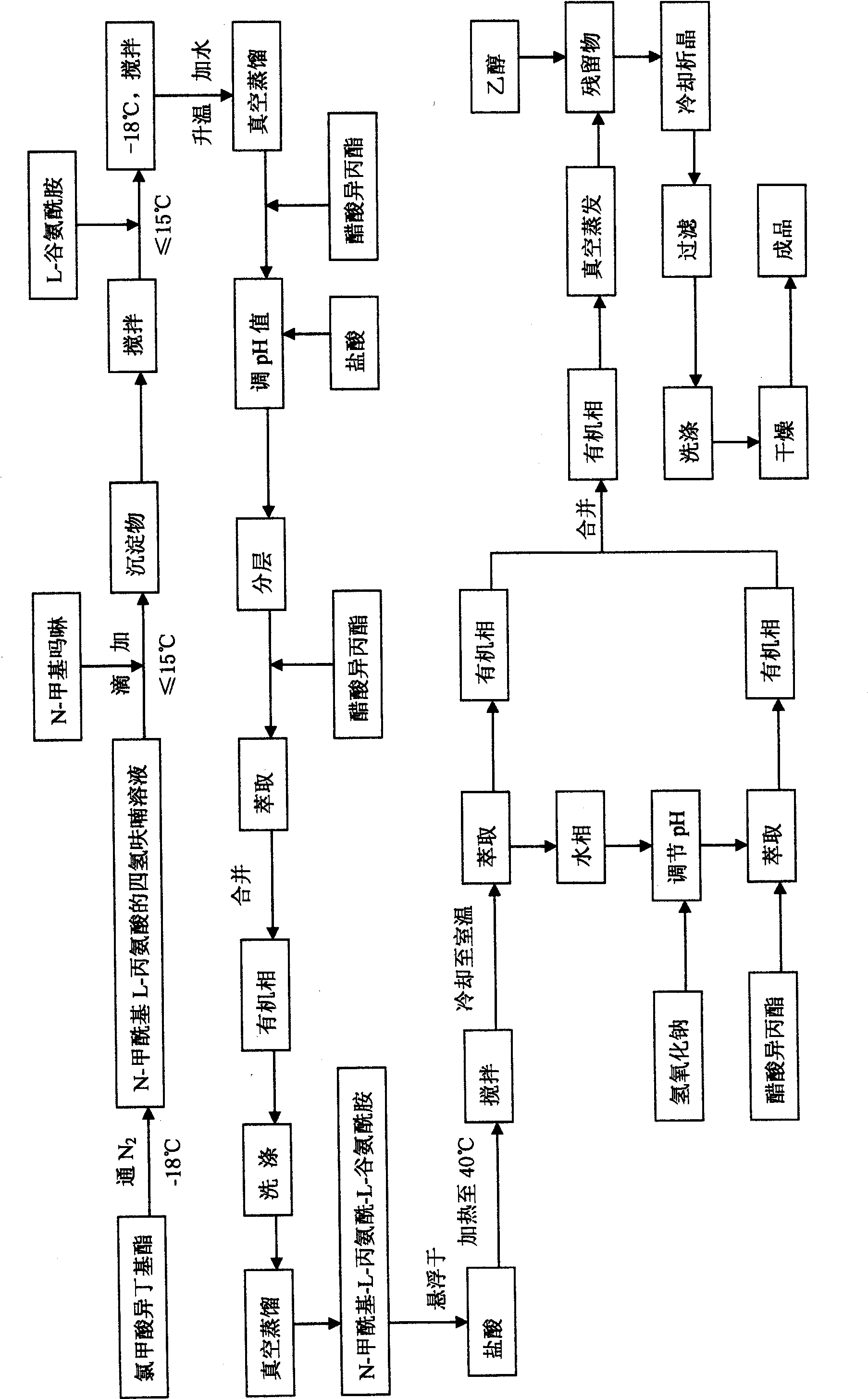
[0025] (1) Under the environment of nitrogen protection at -18°C, 65 grams of isobutyl chloroformate (0.48 moles) were added to 300 milliliters of a solution of 59 grams of N-formyl L-alanine (0.5 moles) in tetrahydrofuran , then 48 gram of N-methylmorpholine (0.48 moles) are added dropwise to the solution, the rate of addition is controlled so that the temperature of the solution is no more than -15°C, a precipitate is formed, and after continuous stirring for 20 minutes, 66 gram of L-glutamine ( 0.45 mol) of tetrahydrofuran solution (300 ml), keep the reaction temperature not exceeding -15°C when adding, and then stir at -18°C for 1 hour. The reaction mixture was warmed up to 0° C., 500 grams of water was added at this temperature, and then tetrahydrofuran was distilled off under vacuum, 300 milliliters of isopropyl acetate was added, and the pH value of the reaction mixture was adjusted to 1.5 with 5 mol / liter hydrochloric acid. After the layers were separated, they were ex...
Embodiment 2
[0030](1) Under the environment of nitrogen protection at -18°C, 86.67 grams of isobutyl chloroformate (0.64 moles) were added to 400 milliliters of a tetrahydrofuran solution of 78.67 grams of N-formyl L-alanine (0.67 moles). , then 64 gram of N-methylmorpholine (0.64 moles) are added dropwise to the solution, the rate of addition is controlled so that the temperature of the solution is no more than -15°C, a precipitate is formed, and after continuous stirring for 20 minutes, 88 gram of L-glutamine ( 0.6 mol) of tetrahydrofuran solution (400 ml), keep the reaction temperature not exceeding -15°C when adding, and then stir at -18°C for 1 hour. The reaction mixture was warmed up to 0° C., 600 grams of water was added at this temperature, tetrahydrofuran was then distilled off under vacuum, 400 milliliters of isopropyl acetate was added, and the pH value of the reaction mixture was adjusted to 1.5 with 1 mol / liter of hydrochloric acid. After the layers were separated, they were ...
Embodiment 3
[0035] (1) Under the environment of nitrogen protection at -18°C, 130 grams of isobutyl chloroformate (0.96 mole) was added to 600 milliliters of a solution of 118 grams of N-formyl L-alanine (1 mole) in tetrahydrofuran , then 96 gram of N-methylmorpholine (0.96 moles) are added dropwise to the solution, the rate of addition is controlled so that the temperature of the solution is no more than -15°C, a precipitate is formed, and after continuous stirring for 20 minutes, 132 gram of L-glutamine ( 0.9 mol) of tetrahydrofuran solution 600ml, keep the reaction temperature not exceeding -15°C when adding, and then stir at -18°C for 1 hour. The reaction mixture was warmed up to 0° C., 1000 grams of water was added at this temperature, tetrahydrofuran was then distilled off under vacuum, 600 milliliters of isopropyl acetate was added, and the pH value of the reaction mixture was adjusted to 1.5 with 3 mol / liter hydrochloric acid. After the layers were separated, they were extracted t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com