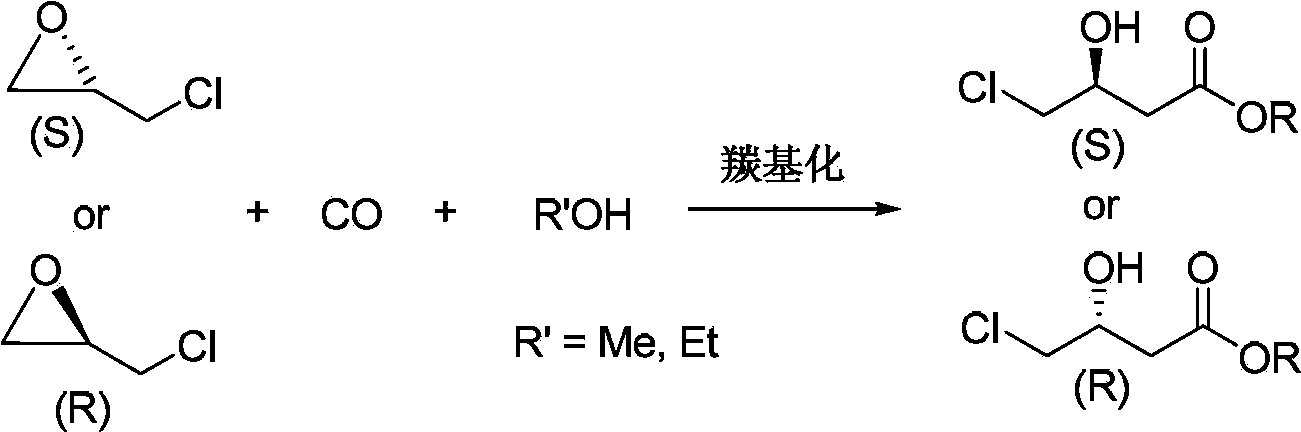
Preparation method for chiral 4-chloro-3-hydroxybutyrate
A hydroxybutyrate, chiral technology, applied in the field of preparation of chiral 4-chloro-3-hydroxybutyrate, can solve the low yield of methyl 4-chloro-3-hydroxybutyrate, operating conditions Harshness and other problems, to achieve the effect of easy realization of reaction conditions, mild reaction conditions and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In an autoclave with a volume of 600 mL, add 200 mL of anhydrous methanol, 200 mmol of (S)-epichlorohydrin (ee>99.9%), 2.5 mmol of dicobalt octacarbonyl, and 10 mmol of ZnBr 2 (pyridine) 2 . Seal the reactor, replace the reactor with carbon monoxide 3 times, and seal the reactor. In the Schlenk vacuum line, the reaction system was replaced three times with carbon monoxide gas at room temperature, and the pressure of CO gas was charged at 6.0 MPa. The temperature was controlled by a temperature controller to slowly rise to 60° C., reacted for 8 hours, cooled to room temperature, and unloaded.
[0034] The liquid obtained by the reaction is qualitatively analyzed by Agilent 6890 / 5973 GC / MS, and quantitatively analyzed by Agilent 7890 gas chromatography: the conversion rate of (S)-epichlorohydrin is 99%, and the conversion rate of (S)-4-chloro-3-hydroxybutyrate Acid methyl ester selectivity 94%. Distilled under reduced pressure to obtain (S)-4-chloro-3-hydroxybutyric ac...
Embodiment 2
[0036] In an autoclave with a volume of 600 mL, add 200 mL of anhydrous methanol, 200 mmol of (R)-epichlorohydrin (ee>99.9%), 2.5 mmol of dicobalt octacarbonyl, and 10 mmol of ZnBr 2 (pyridine) 2 . Close the reactor, in the Schlenk vacuum line, replace the reaction system with carbon monoxide gas three times at room temperature, fill the CO gas with a pressure of 6.0MPa, and slowly increase the temperature to 60°C under the control of the temperature controller, react for 8 hours, and cool to room temperature , Unload the kettle.
[0037] The liquid obtained by the reaction is qualitatively analyzed by Agilent 6890 / 5973 GC / MS, and quantitatively analyzed by Agilent 7890 gas chromatography: the conversion rate of (R)-epichlorohydrin is 99%, and the conversion rate of (R)-4-chloro-3-hydroxybutyrate Acid methyl ester selectivity 96%. Distilled under reduced pressure to obtain (R)-4-chloro-3-hydroxybutyric acid methyl ester 27.8g (yield 96%, purity 99%), gas chromatography (Chi...
Embodiment 3
[0039] Add 200 mL of absolute ethanol, 200 mmol of (S)-epichlorohydrin (ee>99.9%), 2.5 mmol of dicobalt octacarbonyl, and 10 mmol of 3-Hydroxypyridine into an autoclave with a volume of 600 mL. Close the reactor, in the Schlenk vacuum line, replace the reaction system with carbon monoxide gas three times at room temperature, fill the CO gas with a pressure of 6.0MPa, and slowly increase the temperature to 60°C under the control of the temperature controller, react for 8 hours, and cool to room temperature , Unload the kettle.
[0040] The liquid obtained by the reaction is qualitatively analyzed by Agilent 6890 / 5973 GC / MS, and quantitatively analyzed by Agilent 7890 gas chromatography: the conversion rate of (S)-epichlorohydrin is 95%, and the conversion rate of (S)-4-chloro-3-hydroxybutyrate Ethyl acid selectivity 93%. Distilled under reduced pressure to obtain 28.0 g of ethyl (S)-4-chloro-3-hydroxybutyrate (yield 95%, purity 99%), gas chromatography (Chiraldex G-TA column) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com