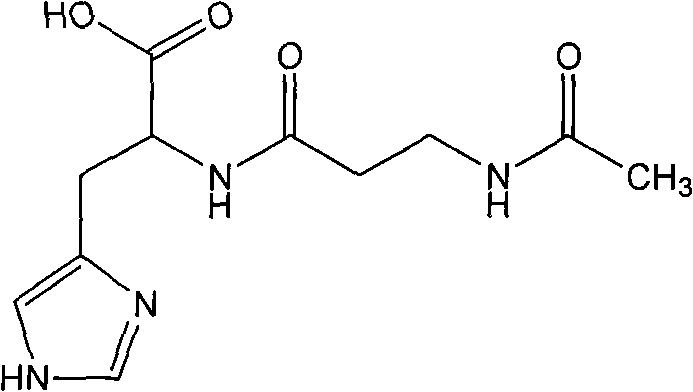
Prepartion method of N-acetyl-L-carnosine
A carnosine and acetyl technology, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of complex synthesis process, low yield and purity, and difficulty in industrial application, and achieves the effects of high yield and ideal purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
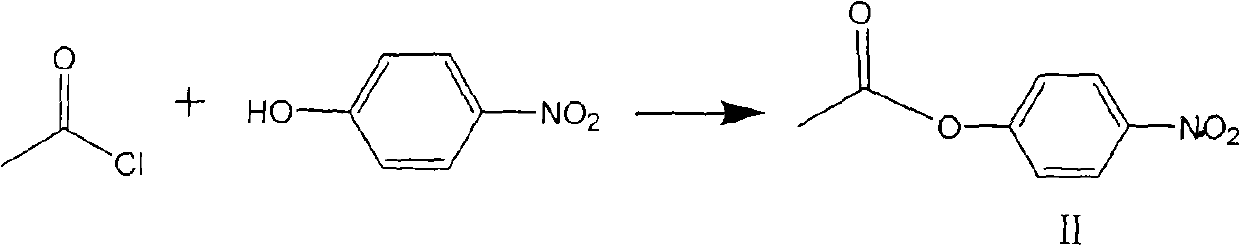
[0020] Prepare active ester solution (II).
[0021] In a 1000ml reaction flask, add 400g tetrahydrofuran, 84g p-nitrophenol (0.604mol) and 61g triethylamine (0.604mol), lower the temperature to 5°C-10°C, add 47.4g acetyl chloride (0.604mol) dropwise for 30 minutes After the dropwise addition, stir again at a temperature of 5° C. to 10° C. for 0.5 hours. Samples are analyzed by HPLC. The raw material p-nitrophenol is completely reacted, filtered, and the filter cake is washed with 20 g of tetrahydrofuran. After merging the filtrates, 520 g of the active ester solution (solution Containing active ester 109g, molar yield 99.6%) wherein, (II) represents active ester.
[0022]
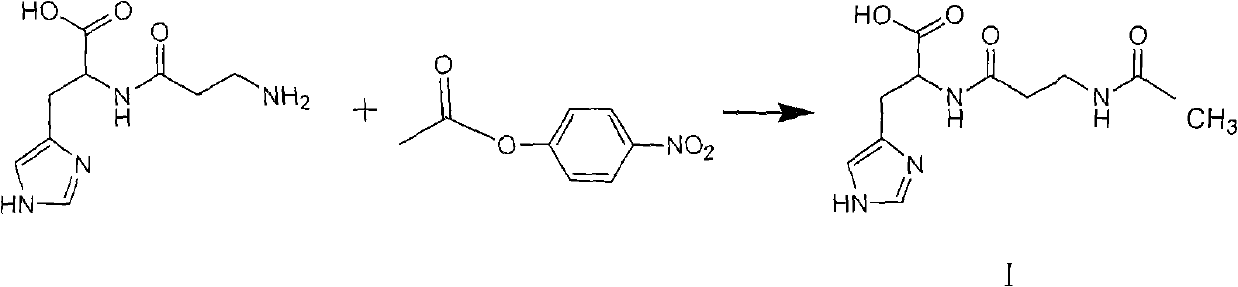
[0023] Preparation of N-acetyl-L-carnosine (I).
[0024] In a 2000ml reaction flask, add 500g of pure water, 113g of L-carnosine (0.5mol) and 68g of imidazole (1mol), stir to dissolve, cool down to 10°C to 15°C, and dropwise add 520g of active ester solution (II) (the solution contains active Ester 109...
Embodiment 2
[0027] Prepare active ester solution (II).
[0028] In a 1000ml reaction flask, add 400g butanone, 84g p-nitrophenol (0.604mol) and 61g triethylamine (0.604mol), cool down to 5°C-10°C, add dropwise 47.4g acetyl chloride (0.604mol), 30 After 10 minutes of dropwise addition, stir again at a temperature of 5°C to 10°C for 0.5 hours. Samples were analyzed by HPLC. The raw material p-nitrophenol was completely reacted, filtered, and the filter cake was washed with 20g methyl ethyl ketone, and the filtrates were combined to obtain 517g active ester solution. (Containing active ester 108g in the solution, molar yield 99.1%)
[0029] Preparation of N-acetyl-L-carnosine (I).
[0030] In a 2000ml reaction flask, add 500g of pure water, 113g of L-carnosine (0.5mol) and 68g of imidazole (1mol), stir to dissolve, cool down to 10°C to 15°C, and dropwise add 517g of active ester solution (II) (the solution contains active Ester 108g, 0.6mol), 60 minutes dropwise, then stirred at a temperat...
Embodiment 3
[0033] Prepare active ester solution (II).
[0034] In a 1000ml reaction flask, add 400g diethyl ether, 84g p-nitrophenol (0.604mol) and 61g triethylamine (0.604mol), cool down to 5°C-10°C, add dropwise 47.4g acetyl chloride (0.604mol) for 30 minutes After the dropwise addition is completed, stir again at a temperature of 5°C to 10°C for 0.5 hours. Samples are analyzed by HPLC. The p-nitrophenol reaction of the raw material is complete, filtered, and the filter cake is washed with 20g of ether, and the combined filtrates are combined to obtain 505g of active ester solution (solution Containing active ester 108g, molar yield 99.1%)
[0035] Preparation of N-acetyl-L-carnosine (I).
[0036] In a 2000ml reaction flask, add 500g of pure water, 113g of L-carnosine (0.5mol) and 68g of imidazole (1mol), stir to dissolve, cool down to 10°C to 15°C, and dropwise add 505g of active ester solution (II) (the solution contains active Ester 108g, 0.6mol), 60 minutes dropwise, then stirred...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com