Method for preparing key intermediate of medicament
A process control and transparent liquid technology, applied in the field of medicine, can solve the problems of difficult industrial production and low product yield, and achieve the effect of high chiral purity and good chemical purity of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
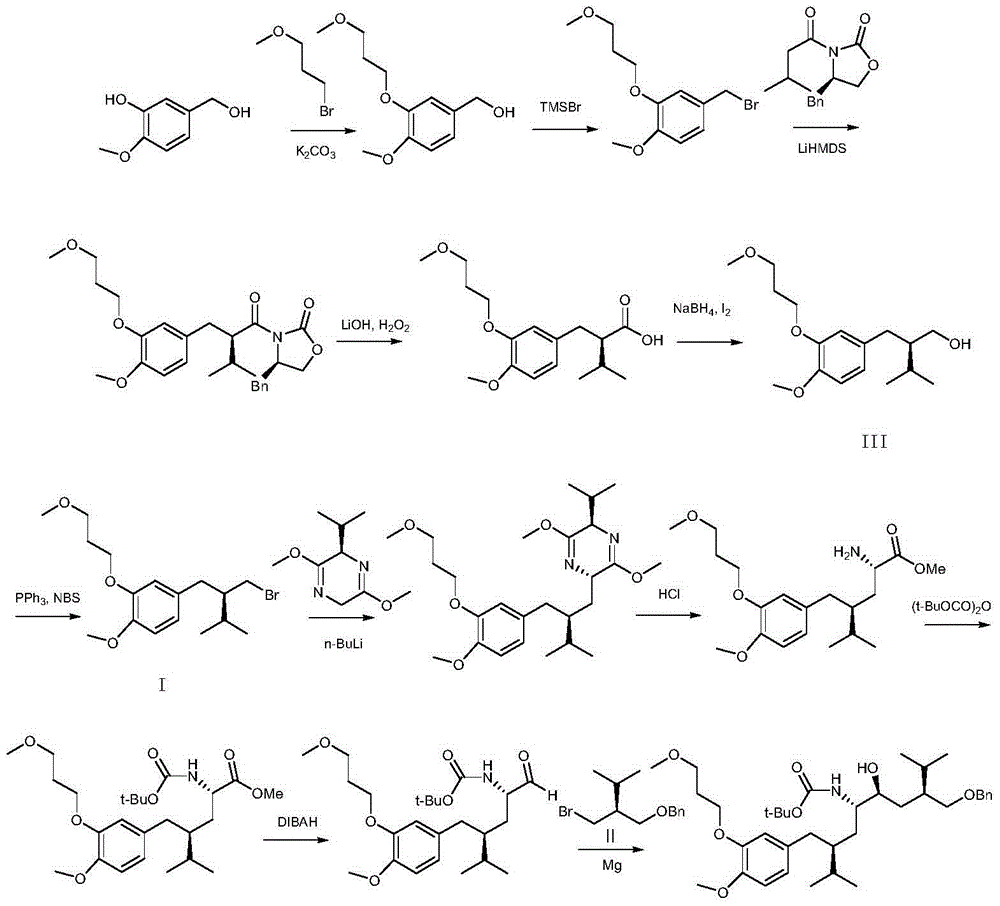
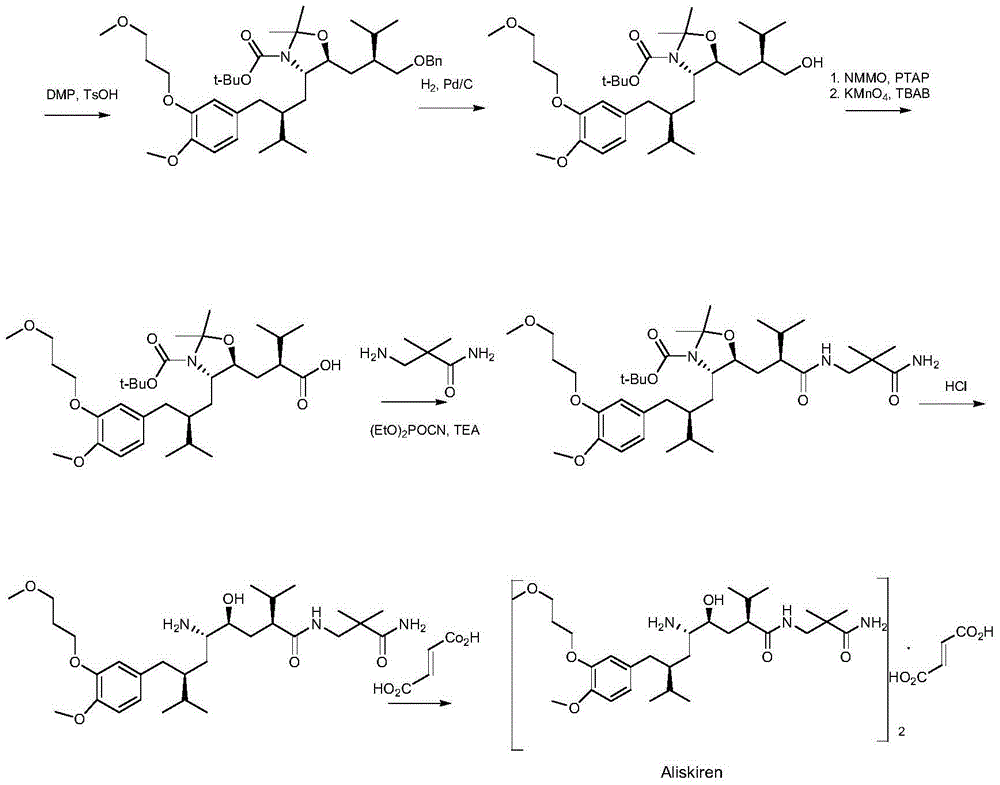
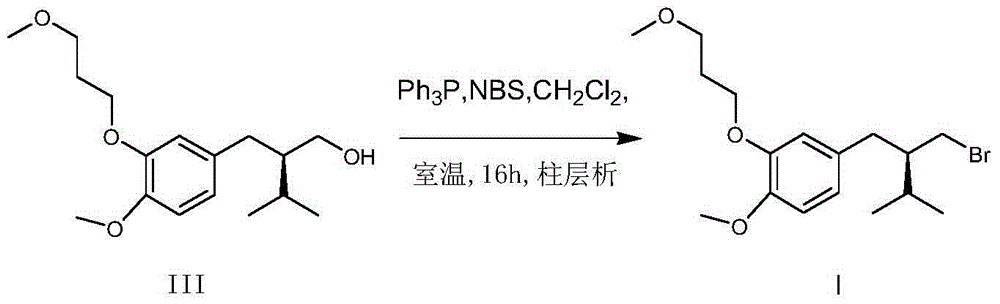
[0027] Synthesis of (R)-2-[4-methoxy-3-(3-methoxypropoxy)benzyl]-3-methylbutyl bromide
[0028] (R-2-[4-methoxy-3-(3-methoxypropoxy)benzyl]-3-methylbutanol (14.82g, 50mmol) dissolved in N,N-dimethylformaldehyde Phosphorus oxybromide (14.33g, 50mmol) was slowly added dropwise to amide (234ml), the temperature was controlled at 20°C during the dropwise addition, and reacted at 20°C for 8 hours after the dropwise addition. Cool to room temperature, and slowly pour water (702ml) , extracted three times with n-hexane, combined the n-hexane layers, washed twice with saturated sodium bicarbonate and saturated sodium chloride respectively, dried the organic layer with anhydrous sodium sulfate, filtered, and concentrated the filtrate to obtain 17g of oily matter. Add methanol 51ml, Crystallize at -10°C, filter, and vacuum-dry the filter cake at room temperature to obtain 14.3 g of white solid, yield 79.6%. mp: 51-53°C, chiral purity above 99.9%. 1 HNMR (ppm, CDCl 3 ):6.72-6.81(m,3H),...
Embodiment 2
[0030] Synthesis of (R)-2-[4-methoxy-3-(3-methoxypropoxy)benzyl]-3-methylbutyl bromide
[0031] (R)-2-[4-methoxy-3-(3-methoxypropoxy)benzyl]-3-methylbutanol (14.82g, 50mmol), N,N-dimethylformaldehyde Amide (0.4g, 5.5mmol) was dissolved in toluene (234ml), and phosphorus oxybromide (15.05g, 52.5mmol) was slowly added dropwise. During the dropping process, the temperature was controlled below 60°C. After the dropwise addition, it was reacted at 80°C for 2 hours. Cool to room temperature, add water (234ml), stir for 20 minutes, separate layers, extract the aqueous layer three times with petroleum ether, combine the organic layers, wash twice with saturated sodium bicarbonate and saturated sodium chloride, and wash the organic layer with anhydrous sulfuric acid It was dried over sodium, filtered, and the filtrate was concentrated to obtain 17.2 g of oil. Add ethyl acetate / n-hexane mixed solvent 17.2ml / 34.4ml, crystallize at -10°C, filter, and vacuum-dry the filter cake at room te...
Embodiment 3
[0033] Synthesis of (S)-2-(benzyloxymethyl)-3-methylbutyl bromide
[0034] (R)-2-(Benzyloxymethyl)-3-methylbutanol (15.6g, 75mmol) was dissolved in N,N-dimethylformamide (246ml), slowly added dropwise phosphorus oxybromide (22.58 g, 78.75mmol), the dropwise addition process controlled the temperature below 50°C, and reacted at 50°C for 3 hours after the dropwise addition. Cool to room temperature, slowly pour into water (246ml), extract three times with n-hexane, combine the n-hexane layers, wash twice with saturated sodium bicarbonate and saturated sodium chloride respectively, dry the organic layer with anhydrous sodium sulfate, filter, The filtrate was concentrated to obtain 18.1 g of oily substance, which was distilled under reduced pressure to obtain 16.6 g of a colorless transparent liquid with a yield of 81.1% and a chiral purity of over 99.9%.
[0035] 1 HNMR (ppm, CDCl3): 7.38-7.24 (m, 5H), 4.52 (s, 2H), 3.70 (dd, J=4.4Hz, 10Hz, 1H), 3.62 (dd, J=4.4Hz, 9.6Hz, 1H ),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com