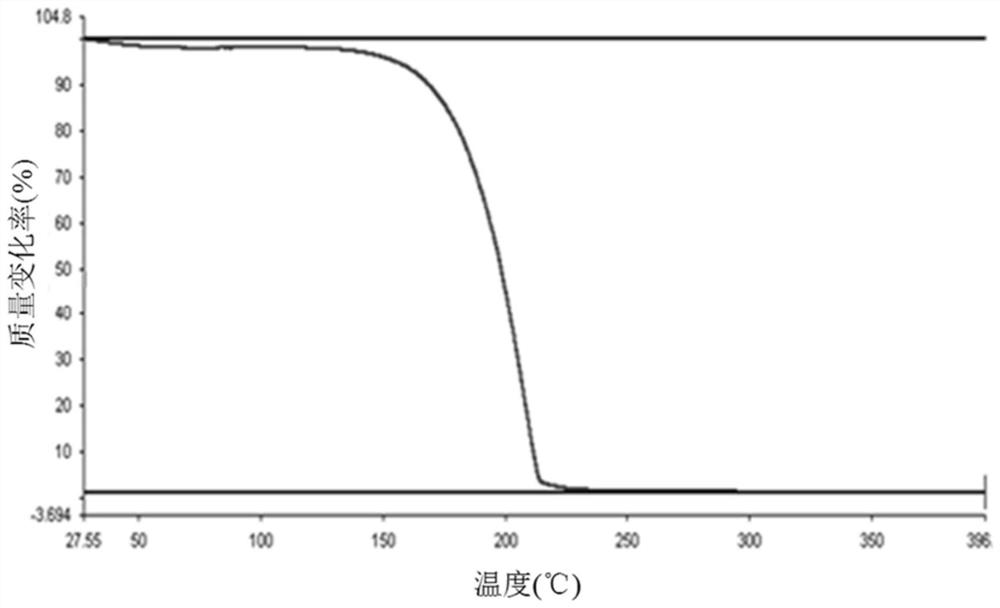
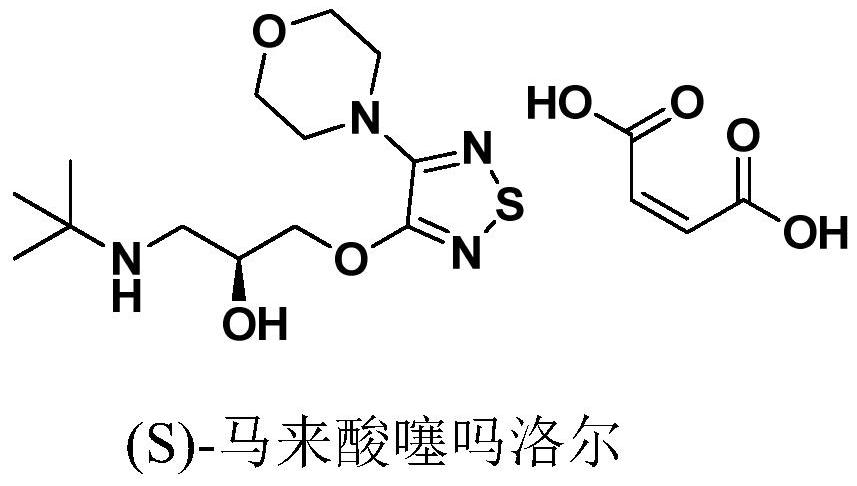
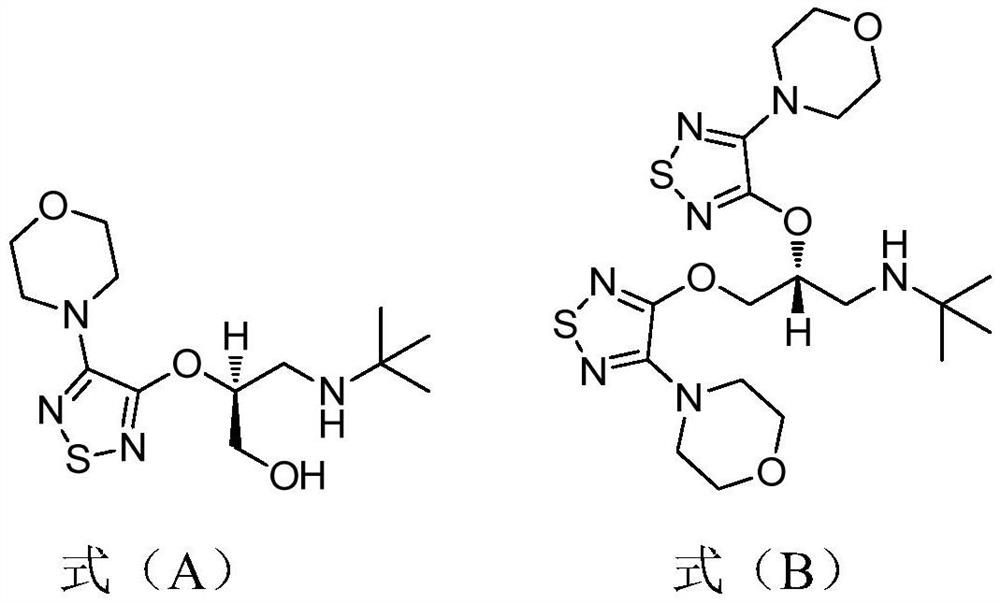
Preparation method of timolol hydrate
The technology of timolol hydrate and timolol maleate is applied in the field of preparation of timolol hydrate, and can solve the problems of complicated S-timolol hydrate process, harsh conditions and unfriendly environment And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 119.50 grams of S-timolol maleate, 720 milliliters of water and 240 milliliters of dichloromethane were added to a three-necked reaction flask, then the temperature was lowered to 0° C. and 10% NaOH aqueous solution was added to adjust the pH of the reaction solution to 14. The dichloromethane phase and the aqueous phase were extracted twice with 240 ml of dichloromethane, and then the dichloromethane phases were combined. After washing the dichloromethane phase with 240 ml of saturated NaCl aqueous solution for 3 times, the dichloromethane phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 84.51 grams of S-timolol oil. The analytical detection results of the chemical purity of the substance are shown in Table 2, and the optical purity detection results are shown in Table 3.
[0049] Table 2
[0050]
[0051]
[0052] As can be seen from the table, the percentage content of S-timolol and R-t...
Embodiment 2
[0069] 213.52 grams of S-timolol maleate, 1500 milliliters of water and 230 milliliters of dichloromethane were added to a three-necked reaction flask, then the temperature was lowered to 0° C. and 10% NaOH aqueous solution was added to adjust the pH of the reaction solution to 14. The dichloromethane phase and the aqueous phase were extracted twice with 230 ml of dichloromethane, and then the dichloromethane phases were combined. After washing the dichloromethane phase with 230 ml of saturated NaCl aqueous solution for 3 times, the dichloromethane phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 154.12 g of S-timolol oil. The analytical detection results of the chemical purity of the S-timolol oil are shown in Table 6, and the optical purity detection results are shown in Table 7.
[0070] Table 6
[0071]
[0072]
[0073] As can be seen from the table, the percentage content of S-timolol and R...
Embodiment 3
[0088] 175.44 grams of S-timolol maleate, 1 liter of water and 350 milliliters of dichloromethane were added to the three-necked reaction flask, then the temperature was lowered to 0° C. and 10% NaOH aqueous solution was added to adjust the pH of the reaction solution to 14. The methyl chloride phase and the aqueous phase were extracted twice with 350 ml of dichloromethane, and then the dichloromethane phases were combined. After washing the dichloromethane phase with 350 ml of saturated NaCl aqueous solution for 3 times, the dichloromethane phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 127.82 g of S-timolol oil. The analytical detection results of the chemical purity of the S-timolol oil are shown in Table 10, and the optical purity detection results are shown in Table 11.
[0089] Table 10
[0090] peak number keep time area high peak width area% Number of theoretical plates ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com