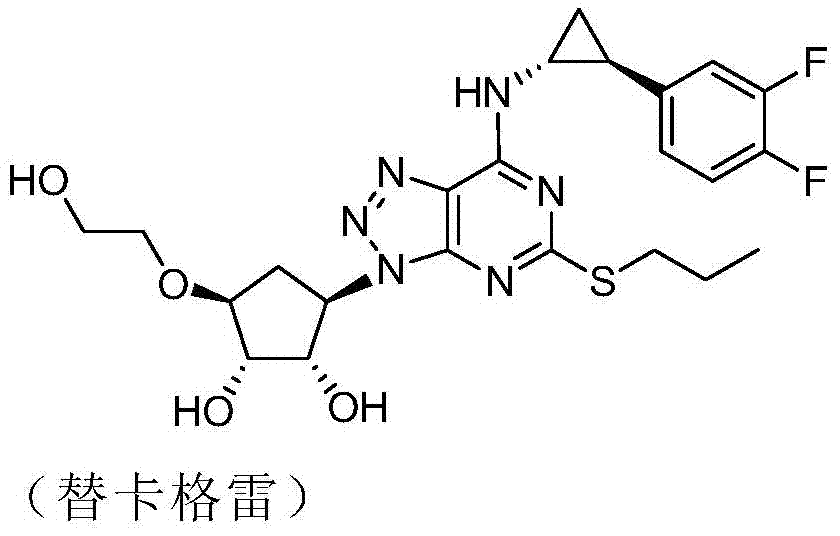
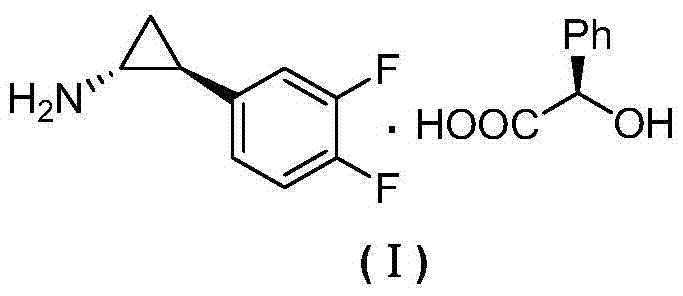
The preparation method of (1r,2s)-2-(3,4-difluorophenyl)cyclopropylamine d-mandelate
A technology of cyclopropanation and triethyl phosphonoacetate, which is applied in the field of medicine and can solve problems such as odor, unfavorable industrial production, and unstable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
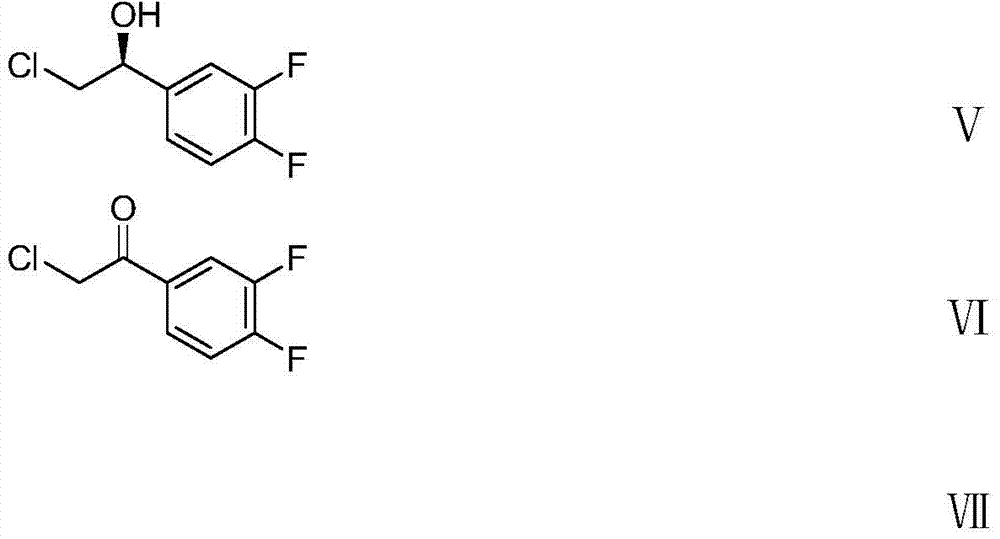
[0066] Preparation of 2-chloro-1-(3,4-difluorophenyl)ethanone (compound Ⅵ)
[0067]
[0068] Add anhydrous aluminum chloride (134.4g, 1.01mol) and dichloromethane (300ml) into a 1L four-necked flask. With mechanical stirring, chloroacetyl chloride (113.9 g, 1.01 mol) was added dropwise at 20-25 ° C, and the drop was completed in about 2 hours. The temperature was raised to reflux, and o-difluorobenzene (100.0 g, 0.88 mol) was slowly added dropwise for about 1 hour. Continue to reflux for 2h. After the reaction solution was cooled to room temperature, it was slowly poured into 600 ml of ice water for quenching. The layers were allowed to stand, and the aqueous layer was extracted with dichloromethane (300ml×2). The organic layers were combined and washed successively with saturated sodium bicarbonate solution (1L) and water (1L). After drying over anhydrous magnesium sulfate, the solvent was evaporated under reduced pressure to obtain compound VI (153.3 g, 91.8%) as a yel...
Embodiment 2-5
[0070] Preparation of 2-chloro-1-S-(3,4-difluorophenyl)-ethanol (compound Ⅴ)
[0071]
[0072] Compound (Ⅵ) reacted at 15°C for 1 hour under the action of borane-dimethyl sulfide complex to generate compound (Ⅴ). The results are shown in Table 1.
[0073] Table 1 The influence of different catalysts on the reaction
[0074] Numbering
[0075] It can be seen from Table 1 that when the substituent is a hydrogen atom, methyl or n-butyl, the (S)-CH 3 -CBS is the catalyst, and the ee value of the product compound (Ⅴ) is higher; choose (S)-CH 3 -CBS as a catalyst for follow-up studies.
Embodiment 6-8
[0077] Preparation of 2-chloro-1-S-(3,4-difluorophenyl)-ethanol (compound Ⅴ)
[0078] Using S-CH 3 -CBS was used as a catalyst, and different borane complexes were used as reducing agents. Compound (Ⅵ) was reacted at 15°C for 1 hour to generate compound (Ⅴ). The results are shown in Table 2.
[0079] The influence of table 2 different reducing agents on the reaction
[0080] Numbering
[0081] It can be seen from Table 2 that when borane-dimethyl sulfide and borane-tetrahydrofuran are used as reducing agents, the ee value of the product is higher, and borane-tetrahydrofuran is selected as the reducing agent for subsequent experiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com