Method for efficiently preparing carfilzomib
A carfilzomib and reaction technology, which is applied in the preparation field of carfilzomib, can solve the problems of reduced yield of target product, mild reaction conditions, high compound price, etc., and achieves excellent atom economy, small molecular weight, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
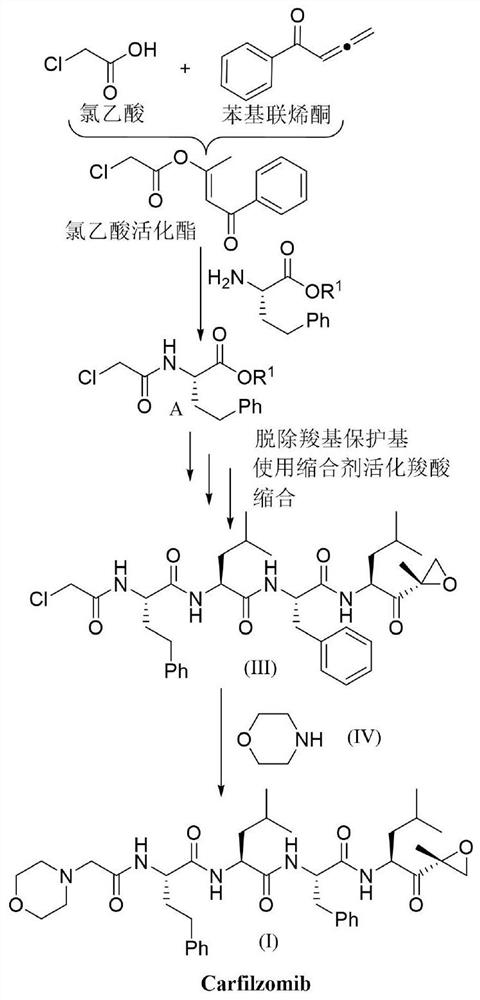
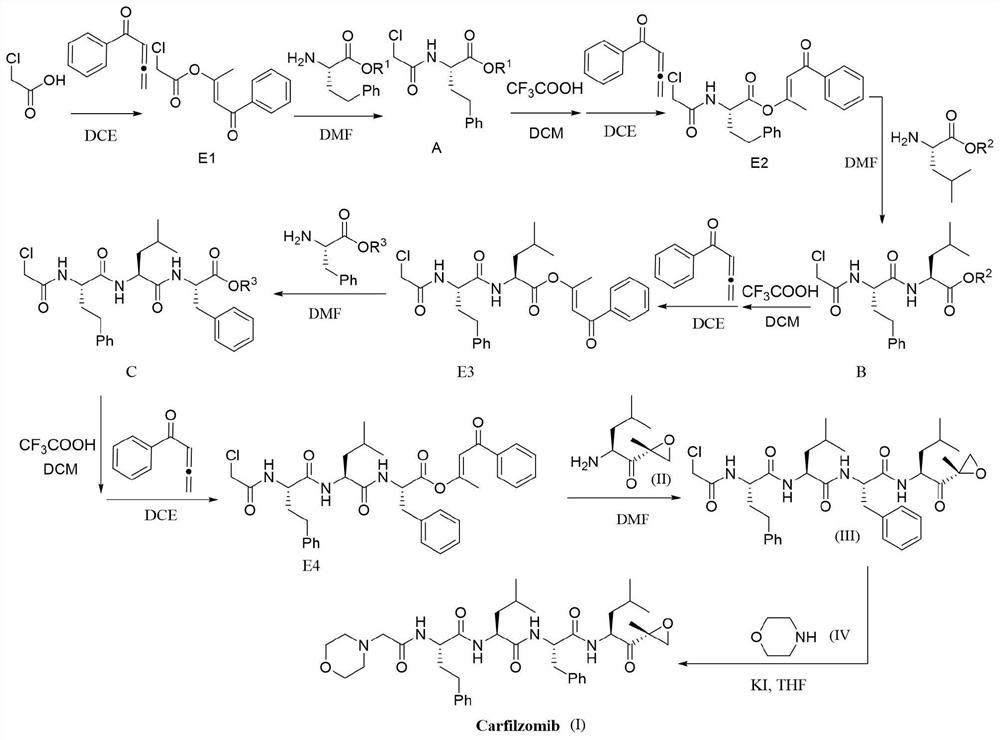
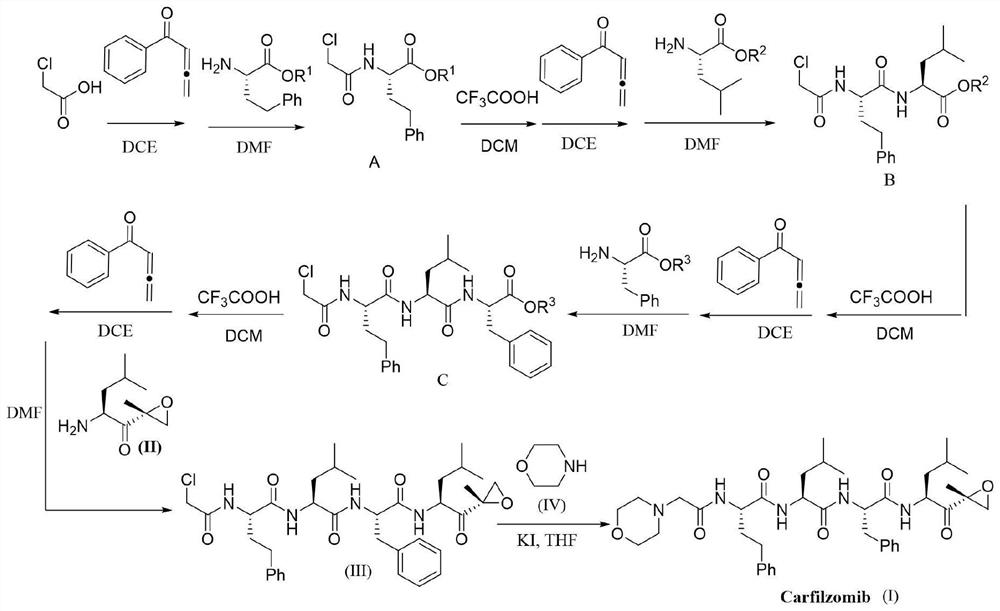
[0094] Synthesis of chloroacetic acid activated ester (E1):
[0095]
[0096] Add chloroacetic acid (5mmol), phenyl allenone (10mmol) and 1,2-dichloroethane (DCE, 10mL) into a clean round-bottomed flask, stir the reaction at 35°C, and track and monitor using TLC, The reaction was complete in 10 hours. After the reaction, the system solution was concentrated and purified by column chromatography to obtain chloroacetic acid activated ester (4.7 mmol) as a light yellow solid with a yield of 94%.
[0097] 1 H NMR (400MHz, CDCl 3)δ7.92(d, J=7.5Hz, 2H), 7.56(t, J=7.4Hz, 1H), 7.47(t, J=7.6Hz, 2H), 6.85(s, 1H), 4.22(s, 2H), 2.42(s, 3H);
[0098] 13 C NMR (100MHz, CDCl 3 )δ190.0, 164.7, 162.7, 138.3, 133.1, 128.6, 128.2, 114.0, 40.8, 18.5.
preparation Embodiment 2
[0100] Synthesis of Coupling Product A:
[0101]
[0102] In a clean round bottom flask, add the activated chloroacetic acid ester (4.5mmol) prepared in Preparation Example 1, H 2 N-HomoPhe-O t Bu (5.4mmol) and N,N-dimethylformamide (DMF, 10mL), the reaction was stirred at room temperature and tracked and monitored by TLC. The reaction was complete within 15min. After the reaction, transfer the reaction solution to a separatory funnel, add water, then add ethyl acetate for extraction, extract three times with ethyl acetate and combine the organic phases, the combined organic phases are dried with anhydrous magnesium sulfate and filtered , the dried organic phase was concentrated and then separated and purified by column chromatography to obtain coupling product A (4.275 mmol), a white solid, with a yield of 95%.
[0103] 1 H NMR (400MHz, CDCl 3 )δ7.25–7.16(m,2H),7.16–7.06(m,3H),7.03(d,J=7.9Hz,1H),4.54–4.44(m,1H),3.94(s,2H),2.67 –2.48(m,2H),2.22–2.08(m,1H),2.03–1.89(m,1...
preparation Embodiment 3
[0106] Synthesis of activated ester E2:
[0107]
[0108] Add coupling product A (4.2mmol), dichloromethane (10mL) and trifluoroacetic acid (10mL) prepared in Preparation Example 2 to a clean round bottom flask, stir at room temperature, and detect by TLC and LC-Ms , The reaction is completed in about 1h. Then, after removing dichloromethane and trifluoroacetic acid under vacuum, add ethyl acetate to the round bottom flask and transfer to a separatory funnel, then add water, extract three times with ethyl acetate and combine the organic phases, the combined organic phases After drying with anhydrous magnesium sulfate and filtering, the dried organic phase was concentrated in vacuo to remove ethyl acetate, and then phenylketene (5 mmol) and 1,2-dichloroethane (DCE, 10 mL ), the reaction was stirred at 35° C., and monitored by TLC, and the reaction was complete in about 15 hours. After the reaction, the solution in the reaction system was concentrated under vacuum and separ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com