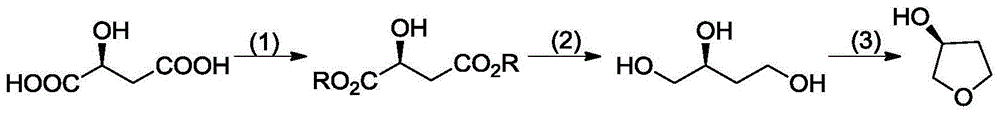Synthetic method of (S)-(+)-3-hydroxytetrahydrofuran
A technology of hydroxytetrahydrofuran and synthesis method, applied in directions such as organic chemistry, can solve problems such as difficulty in meeting purity requirements, difficulty in industrialized production, etc., and achieve the effects of short synthetic route steps, easy removal, and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Add 100g of L-malic acid and 650ml of methanol into a three-neck flask, add 1ml of concentrated sulfuric acid under stirring, heat up to 60-70°C and reflux for 10h. Cool, add saturated aqueous sodium bicarbonate dropwise until the pH value reaches 7-8, concentrate, add water, extract with ethyl acetate, wash the organic phase with water, dry, and concentrate to obtain 103.0 g of a colorless liquid with a yield of 85.2% and a purity of 98.9%; HNMR (CDCl 3 , 300MHz) δ: 4.47(d,1H), 3.76(s,3H), 3.67(s,3H), 3.19(br,1H), 2.71~2.88(m,2H).
[0019] (2) Add 500ml of ethanol and 103g of sodium borohydride to the three-necked flask, and control the temperature at 0 to 10°C under stirring. Add 103.0g of the ethanol (250ml) solution of the product in step 1 dropwise, and drop it within 90min. React at 25°C for 12h, then heat up to 70-80°C and reflux for 8h. Cool, concentrate until there is no solvent, add 2h methanol to the residue, add 10% methanolic hydrochloric acid solutio...
Embodiment 2
[0022] (1) Add 100g of L-malic acid and 300ml of methanol into the three-necked flask, cool to -10°C ~ 0°C, add 120ml of thionyl chloride dropwise under stirring, drop for 2h, stir at room temperature for 4h, then heat up to 60°C React at ~70°C for 1 h, cool, concentrate, add saturated aqueous sodium bicarbonate solution dropwise to the residue until the pH value reaches 7-8, concentrate, add water, extract with ethyl acetate, wash the organic phase with water, dry, and concentrate to obtain a colorless liquid 106.9g, yield 88.4%, purity 97.9%; HNMR (CDCl 3 , 300MHz) δ: 4.47(d,1H), 3.76(s,3H), 3.67(s,3H), 3.19(br,1H), 2.71~2.88(m,2H).
[0023] (2) Add ethanol 500ml ethanol, 103g sodium borohydride to the three-necked flask, control the temperature at 0-10°C under stirring, add dropwise 106.9g of the ethanol (250ml) solution of the product of step 1, drop it within 90min, and finish it in 20-10℃ React at 25°C for 12h, then heat up to 70-80°C and reflux for 8h. Cool, concentra...
Embodiment 3
[0026] (1) Add 100g of L-malic acid and 700ml of isopropanol into a three-necked flask, add 1ml of concentrated sulfuric acid while stirring, heat up to 60-70°C and reflux for 10h. Cool, add saturated aqueous sodium bicarbonate dropwise until the pH value reaches 7-8, concentrate, add water, extract with ethyl acetate, wash the organic phase with water, dry, and concentrate to obtain 133.2 g of a colorless liquid with a yield of 81.9% and a purity of 98.7%; HNMR (CDCl 3 , 300MHz) δ: 4.47(d,1H), 3.57(m,1H), 3.19(br,1H), 2.71~2.88(m,2H), 1.16(d,6H).
[0027] (2) Add 500ml of ethanol and 103g of sodium borohydride to the there-necked flask, and control the temperature at 0 to 10°C under stirring, add dropwise 133.2g of the ethanol (250ml) solution of the product in step 1, drop it within 90min, and finish it in 20~10℃. React at 25°C for 12h, then heat up to 70-80°C and reflux for 8h. Cool, concentrate until there is no solvent, add 2h methanol to the residue, add dropwise 10% m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com