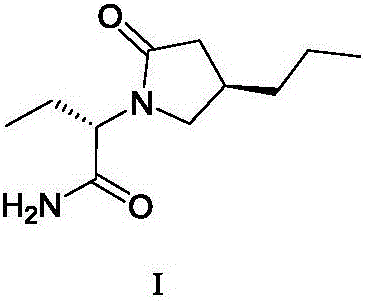
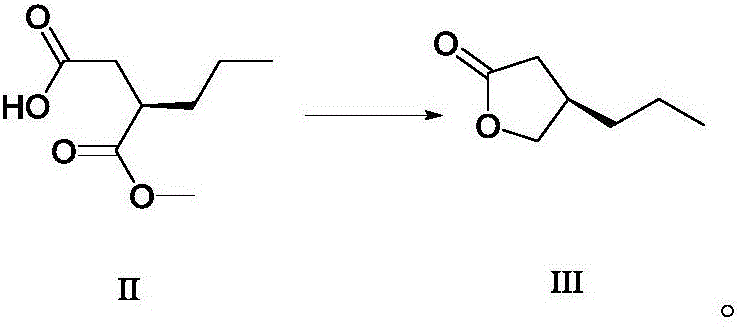
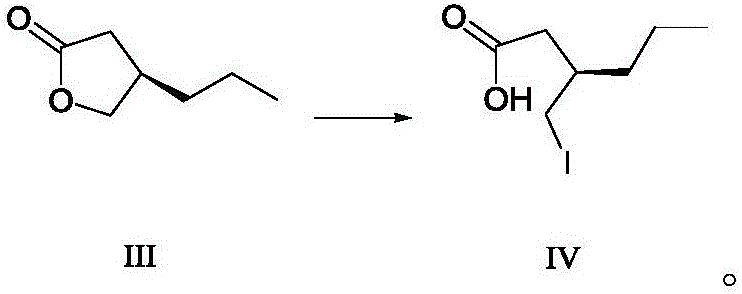
Method for preparing furanone compounds
A compound, the technology of furanone, which is applied in the field of preparation of furanone compounds, can solve the problems of low product purity, low total yield, cumbersome post-processing steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066]
[0067] (R)-3-methoxycarbonylhexanoic acid (174.1 g, 1 mol, ee value 99.2%) was dissolved in 500 mL of methanol, cooled to 0 °C, added with 500 mL of water, cooled to 0 °C, followed by powdered chlorine Calcium chloride (115.8 g, 1.1 mol) and sodium borohydride in ethanol (2M, 800 mL). The reaction solution was stirred at (20°C ~ 30°C) overnight (about 12 hours), hydrochloric acid was added to quench the reaction (6M, 1000 mL), concentrated under reduced pressure, diluted with 500 mL of water, extracted with dichloromethane (3×150 mL), combined The organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain 108.9 g of furanone compound III, yield 85.0%, purity: 97.2% (GC).
[0068] R-3-methoxycarbonylhexanoic acid can be prepared according to the method described in Angewandte Chemie International Edition, 1998, 37(13-14), 1931-1933, and the ee value is greater than 99.0%.
Embodiment 2
[0070]
[0071] Under nitrogen protection, furanone compound III (128.1 g, 1 mol) was dissolved in 1 L of dichloromethane, cooled to 0 °C, trimethylsilyl iodide (150 mL) was added, and the reaction solution was stirred at 20 to 30 °C for 2 Hour. Then add hydrochloric acid solution (1M, 800mL) and sodium thiosulfate aqueous solution (mass percent is 10%, described mass percent refers to the percentage of the quality of sodium thiosulfate and the total mass of sodium thiosulfate aqueous solution, 400mL.) , the aqueous phase was extracted with 1 L of dichloromethane, the organic phase was washed twice with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain brivaracetam intermediate IV (254.6 g), yield 99.5%, purity : 95.6% (GC).
Embodiment 3
[0073]
[0074] Under nitrogen protection, brivaracetam intermediate IV (1280.4 g, 4 mol) was dissolved in 1500 mL of toluene, thionyl chloride (951.8 g, 8 mol) was slowly added, and the reaction solution was stirred at room temperature (20 ℃ ~ 30 ℃) For 24 hours, the solvent was concentrated under reduced pressure. The residue was rectified under reduced pressure with a vacuum pump (0.32 mmHg, 90-95° C.) to obtain 1310 g of compound V as a pale yellow transparent liquid.
[0075] 1310 g of the obtained compound V was dissolved in 2.5 L of dichloromethane solution. Then the above solution was added containing L-2-aminobutanamide hydrochloride (428.9g, 4.2mol), 4A molecular sieve (500g), potassium hydroxide (500g), anhydrous sodium sulfate (500g), tetrabutyl bromide In the dichloromethane solution (12.5L) of ammonium (49g, 0.14mol), the reaction solution was stirred at 20-30°C for 18 hours, then diatomaceous earth was added for filtration, and the filtrate was concentrated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com