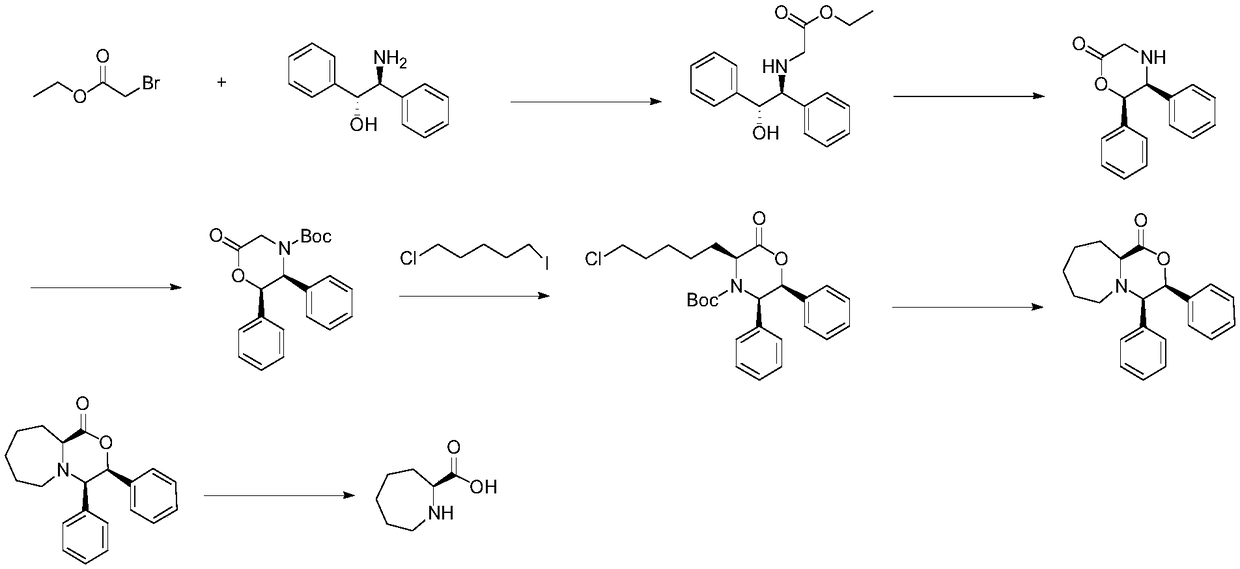
Polybasic nitrogen heterocyclic non-natural chiral amino acid and synthesis method thereof
A technology of chiral amino acid and synthesis method, which is applied in the field of chiral drug synthesis, can solve the problems of high synthesis cost, complex process, and low ee value, and achieve the effect of various types, simple process, and mature process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
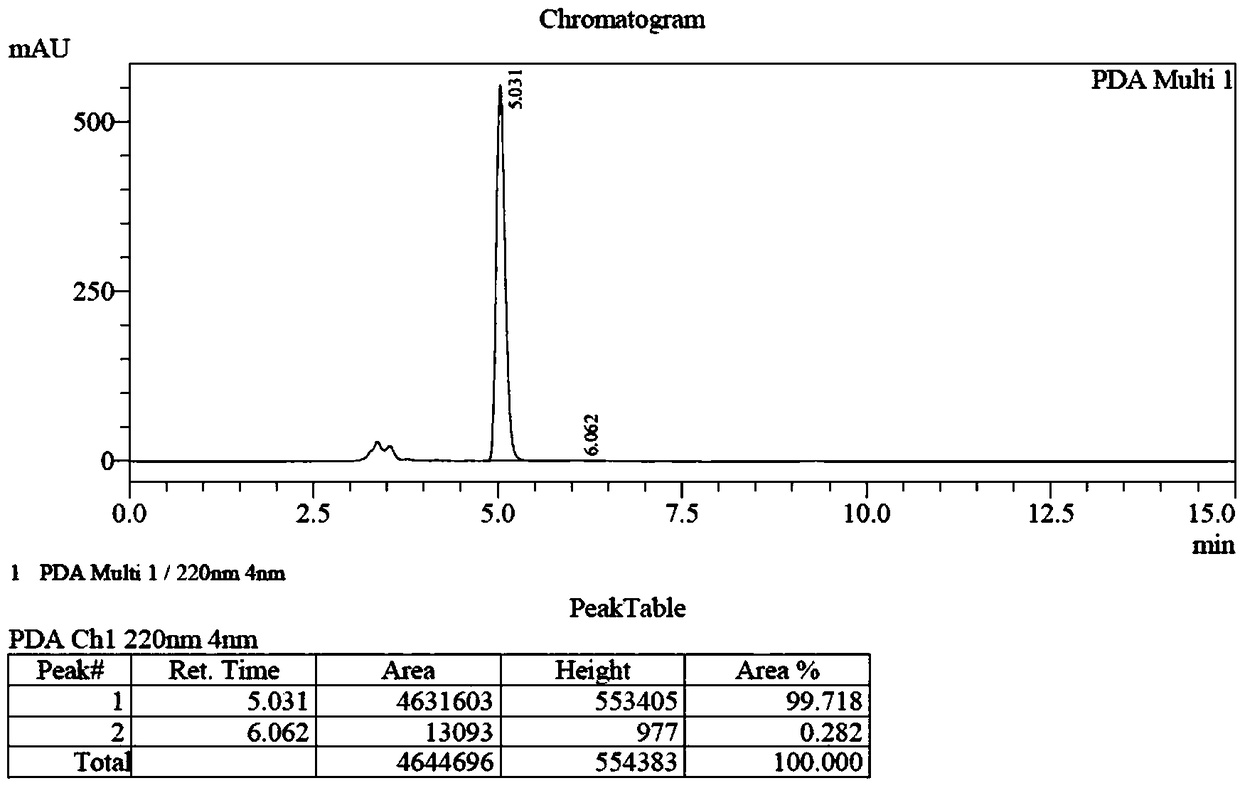
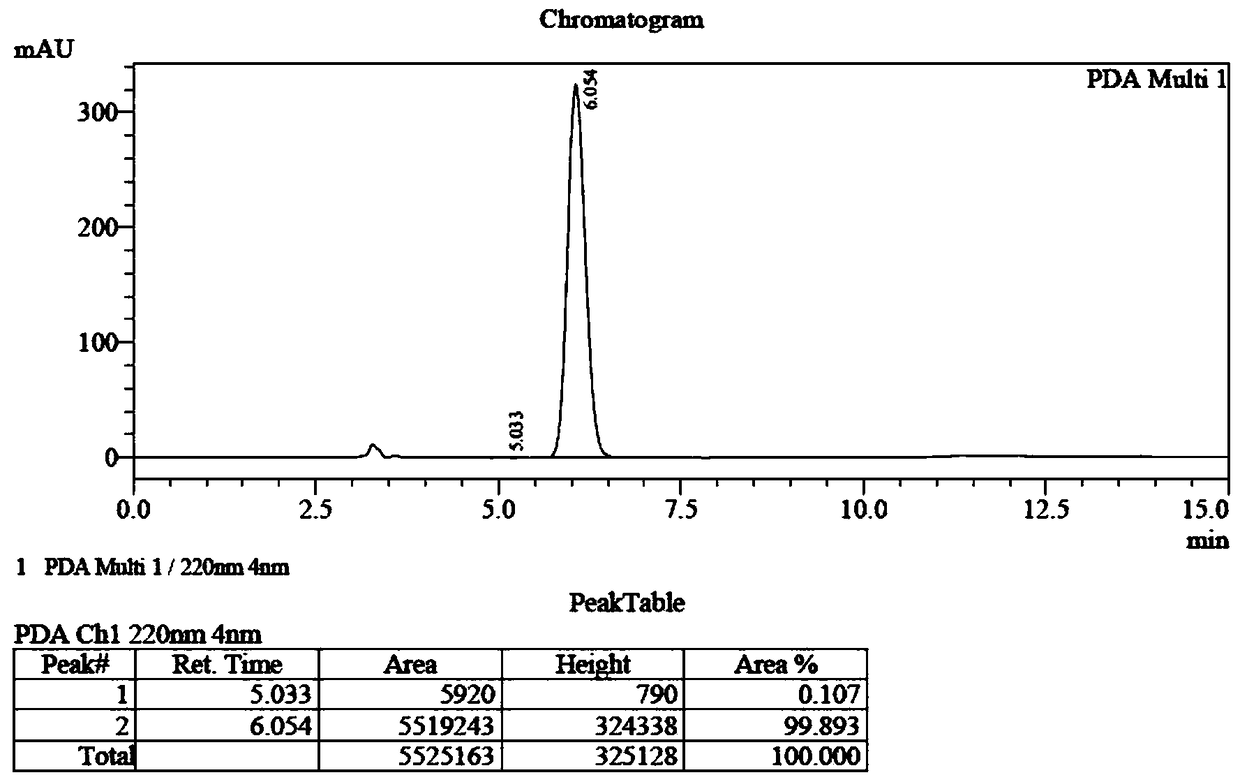
[0062] Example 1 fully embodies the synthesis process of (S)-ethyleneimino-2-carboxylic acid and (R)-ethyleneimino-2-carboxylic acid.
[0063] Example 1:
[0064] (1), the synthesis of 2-Boc-diethyl aminomalonate
[0065]
[0066] Compound 1 (450g, 2.13mol) was dissolved in 1L of dichloromethane, and triethylamine (646g, 6.39mol) was added dropwise in an ice-water bath, and the dropwise reaction was completed at room temperature for 0.5h; under cooling in an ice-water bath, Boc was slowly added dropwise Acid anhydride (512 g, 2.34 mol) was added dropwise and stirred overnight at room temperature. TLC showed that compound 1 disappeared, the reaction solution was filtered, the filter cake was washed with dichloromethane, and the filtrate was concentrated to obtain an oil. The oil was dissolved in ethyl acetate, washed with 1N hydrochloric acid aqueous solution, and then washed with saturated brine, the organic phase was dried over anhydrous sodium sulfate, filtered, and t...
Embodiment 2
[0092] Example 2 fully embodies the synthesis process of (S)-cycloheximino-2-carboxylic acid and (R)-cycloheximino-2-carboxylic acid.
[0093] Example 2:
[0094] (1), the synthesis of 2-Boc-diethyl aminomalonate
[0095]
[0096] Compound 1 (450g, 2.13mol) was dissolved in 1L of dichloromethane, and triethylamine (646g, 6.39mol) was added dropwise in an ice-water bath, and the dropwise reaction was completed at room temperature for 0.5h; under cooling in an ice-water bath, Boc was slowly added dropwise Acid anhydride (512 g, 2.34 mol) was added dropwise and stirred overnight at room temperature. TLC showed that compound 1 disappeared, the reaction solution was filtered, the filter cake was washed with dichloromethane, and the filtrate was concentrated to obtain an oil. The oil was dissolved in ethyl acetate, washed with 1N hydrochloric acid aqueous solution, and then washed with saturated brine, the organic phase was dried over anhydrous sodium sulfate, filtered, and t...
Embodiment 3
[0122] Example 3 fully embodies the synthesis process of (S)-cycloundecimino-2-carboxylic acid and (R)-cycloundecimino-2-carboxylic acid.
[0123] Example 3:
[0124] (1), the synthesis of 2-Boc-diethyl aminomalonate
[0125]
[0126] Compound 1 (500g, 2.36mol) was dissolved in 1L of dichloromethane, and triethylamine (716g, 7.08mol) was added dropwise in an ice-water bath. Acid anhydride (567g, 2.60mol) was added dropwise and stirred at room temperature overnight. TLC showed that compound 1 disappeared, the reaction solution was filtered, the filter cake was washed with dichloromethane, and the filtrate was concentrated to obtain an oil. The oil was dissolved in ethyl acetate, washed with 1N hydrochloric acid aqueous solution, and then washed with saturated brine, the organic phase was dried over anhydrous sodium sulfate, filtered, and the organic phase was concentrated to obtain 578 g of yellow oil compound 2 (yield: 89%). The next reaction was carried out directly w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com