Preparation and purification method of pantoprazole optical antimer
A technology of pantoprazole and a purification method, which is applied in the direction of organic chemistry, etc., can solve the problems of high cost of pantoprazole sodium optical antipodes, insufficient optical purity, and influence on the purity of salt formation, so as to improve synthesis efficiency, The effect of shortening the reaction time and increasing the production yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
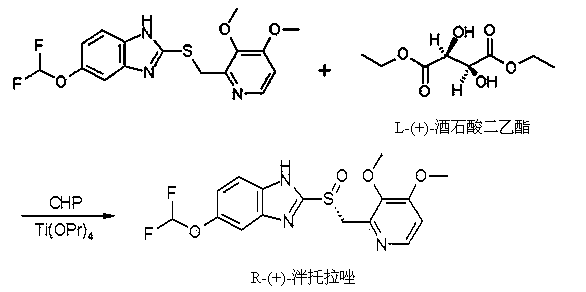
[0035] Embodiment 1: the preparation of L-pantoprazole
[0036] In a 10L reactor, put in 4.8L of toluene, and put in 5-difluoromethoxy-2-[[(3,4-dimethoxy-2-pyridyl)methyl]sulfur]-1H-benzene under stirring and imidazole 1kg, heated to 70°C in a jacketed water bath and stirred to dissolve. Cool in a water bath to 50°C, add 374 g of D-diethyl tartrate (DET) dropwise, and keep stirring for 20 minutes. Add 7ml of water and 256g of tetraisopropyl titanate, immediately stop stirring and let it stand for 40 minutes, cool down to 25-26°C in a jacketed water bath, add 153ml of N,N-diisopropylethylamine dropwise, and then add peroxide Hydrogen cumene (CHP, concentration 80%) 660g, temperature control 28~30℃, about 1 hour. The addition was complete and stirred for an additional 2 hours. Filter and wash the filter cake with 3L of methyl tert-butyl ether. Filter, drain, and dry at room temperature to obtain 656 g of off-white solid powder, which is the crude product of L-pantoprazole. ...
Embodiment 2
[0038] Embodiment 2: the preparation of L-pantoprazole
[0039]In a 10L reactor, put 7.5L of toluene into it, and put 5-difluoromethoxy-2-[[(3,4-dimethoxy-2-pyridyl)methyl]sulfur]-1H-benzene under stirring and imidazole 1kg, heated to 70°C in a jacketed water bath and stirred to dissolve. Cool in a water bath to 50°C, add 374 g of D-diethyl tartrate (DET) dropwise, and keep stirring for 20 minutes. Add 7ml of water, 256g of tetraisopropyl titanate, stop stirring immediately and let it stand for 40 minutes, cool down to 25-26°C in a jacketed water bath, add 153ml of N,N-diisopropylethylamine dropwise, and then add peroxide Hydrogen cumene (CHP, concentration 85%) 621g, temperature control 28~30℃, about 1 hour. The addition was complete and stirred for an additional 2 hours. After filtering, the filter cake was beaten and washed with 4L of methyl tert-butyl ether. Filter, drain, and dry at room temperature to obtain 685 g of off-white solid powder, which is the crude product...
Embodiment 3
[0041] Embodiment 3: the preparation of dexpantoprazole
[0042] In a 10L reactor, put in 5.5L of toluene, and put in 5-difluoromethoxy-2-[[(3,4-dimethoxy-2-pyridyl)methyl]sulfur]-1H-benzene under stirring and imidazole 1kg, heated to 70°C in a jacketed water bath and stirred to dissolve. Then it was cooled to 50° C. in a water bath, 374 g of L-diethyl tartrate was added dropwise, and the mixture was stirred and kept for 20 minutes. Add 7ml of water, 256g of tetraisopropyl titanate, stop stirring immediately and let it stand for 40 minutes, cool down to 25-26°C in a jacketed water bath, add 153ml of N,N-diisopropylethylamine dropwise, and then add peroxide Hydrogen cumene (CHP, concentration 80%) 660g, temperature control 28~30℃, about 1 hour. The addition was complete and stirred for an additional 2 hours. After filtering, the filter cake was beaten and washed with 3.5 L of methyl tert-butyl ether. Filter, drain, and dry at room temperature to obtain 664 g of off-white so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com