Method for preparing and purifying (L)-pantoprazole sodium
A technology of levo-pantoprazole sodium and pantoprazole, which is applied in the field of preparation and purification of levo-pantoprazole sodium, can solve problems such as inapplicability to large-scale production, cumbersome post-processing, and influence on the purity of salt formation, and achieve reaction Mild conditions, high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of L-pantoprazole
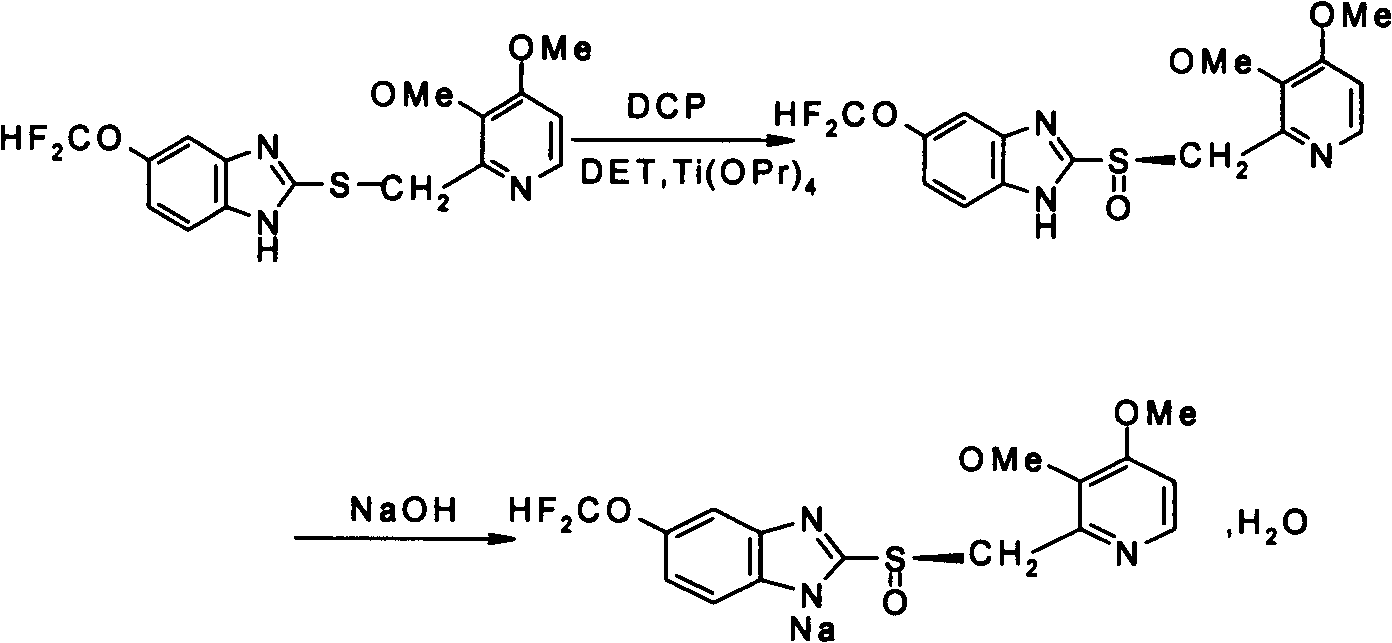
[0029] Add 1kg of 5-difluoromethoxy-2-[[(3,4-dimethoxy-2-pyridyl)methyl]sulfur]-1H-benzimidazole, 7.5L of toluene, D-diethyl tartrate (DET) 374g, tetraisopropyl titanate 256g and appropriate amount of water (the water content in the reaction system is about 7ml), stir and heat up to 60-65°C to form a transparent solution, keep stirring for 1 hour, the reaction solution Cool down to 5-10°C, add 153ml of N,N-diisopropylethylamine, then slowly add 1kg of dicumyl hydroperoxide (DCP, concentration 80%) dropwise three times, react at -2-0°C for 20 Hours, use HPLC to trace the residue of raw materials to less than 1% as the end point of the reaction. After the reaction, filter the reaction solution, wash the filter cake with methyl tert-butyl ether, and dissolve the washed white powder solid in 3L 2% NaOH Add 2% medicinal charcoal to the solution, stir for 20 minutes to decolorize, and filter to remove medicinal charcoal. The...
Embodiment 2
[0031] Embodiment 2: the preparation of L-pantoprazole
[0032] Add 5-difluoromethoxy-2-[[(3,4-dimethoxy-2-pyridyl)methyl]sulfur]-1H-benzimidazole 1kg, ethyl acetate 7.5 L, D-diethyl tartrate (DET) 374g, tetraisopropyl titanate 256g and water 10ml, stir and heat up to 60-65°C to form a transparent solution, keep stirring for 1 hour, and cool the reaction solution to 5-10°C, Add 153ml of N,N-diisopropylethylamine, then slowly add 1kg of dicumyl hydroperoxide (DCP, concentration 80%) dropwise three times, react at -5-0°C for 24 hours, and follow up to the raw material by HPLC The residue is reduced to less than 1% as the end point of the reaction. After the reaction is over, filter the reaction solution, wash the filter cake with methyl tert-butyl ether, dissolve the washed white powder solid in 3L 2% NaOH solution, add 2% Medicinal charcoal was stirred for 20min for decolorization, and the medicinal charcoal was removed by filtration. The filtrate was adjusted to pH 9-10 with...
Embodiment 3
[0034] Embodiment 3: the preparation of L-pantoprazole sodium
[0035] Suspend 0.56 kg of dry L-pantoprazole in 1.4 L of isopropanol, add dropwise sodium hydroxide solution (67.8 g of sodium hydroxide dissolved in 67.8 ml of water) at 25-30° C., and stir until the solution becomes clear. Add 6L of methyl tert-butyl ether, lower the temperature to -5~0°C and stir for 2 hours to precipitate a solid, filter, wash the filter cake with 3L of methyl tert-butyl ether, and dry at 40~45°C for 12 hours under reduced pressure (93kPa) , 0.51kg of white crystalline powder was obtained, the yield was 89.2%, melting point: 146.8~147.5°C, [a] D 20 =-121.7 (acetonitrile:methanol=1:1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com