Preparation method for chiral 4-cyano-3-hydroxybutyrate
A hydroxybutyrate and chiral technology, which is applied in the field of preparation of chiral 4-cyano-3-hydroxybutyrate, can solve the problem that chiral ruthenium-phosphine ligands or enzymes are difficult to prepare and the product purity cannot meet the requirements , the difficulty of obtaining substrates, etc., the equipment requirements and reaction conditions are easy to achieve, suitable for large-scale production, and the cost is low.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
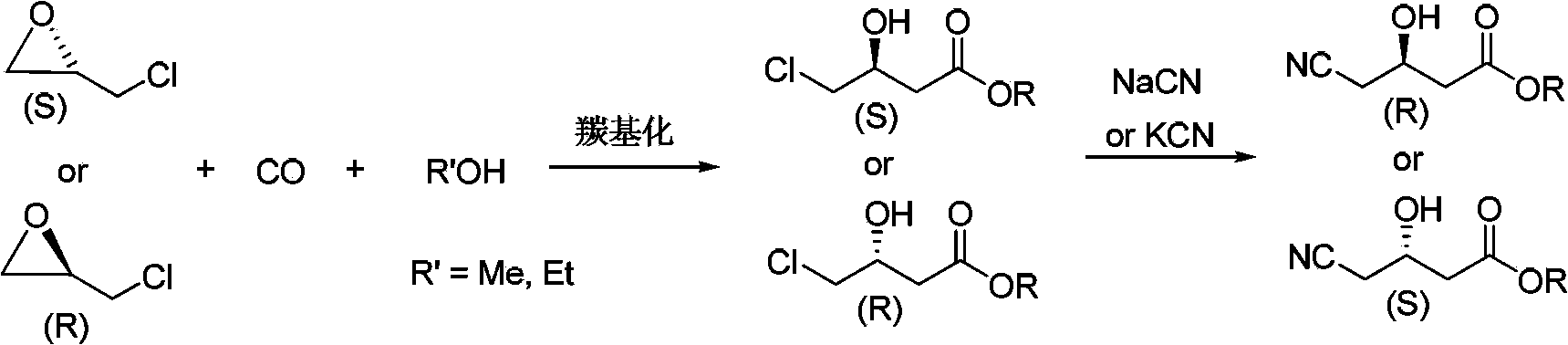
[0029] The embodiment of the present invention discloses a preparation method of chiral 4-cyano-3-hydroxybutyrate, comprising the following steps:
[0030] (a) Add chiral epichlorohydrin, alcohol, and catalyst into the autoclave, feed in carbon monoxide, and conduct a heating reaction. After the reaction, refine to obtain chiral 4-chloro-3-hydroxybutyrate;
[0031] (b) adding a cyanide reagent to chiral 4-chloro-3-hydroxybutyrate for cyanation to obtain chiral 4-cyano-3-hydroxybutyrate.
[0032] The route of above-mentioned preparation method can be expressed as follows:
[0033]
[0034] In the above preparation method, due to the advanced oxo synthesis technology, the synthesis of chiral 4-chloro-3-hydroxybutyrate is realized through a one-step reaction, which meets the current development requirements of green fine chemical industry; the required raw material chiral epoxy Chloropropane rings, alcohols, and carbon monoxide are bulk chemicals, easy to obtain, and low in c...
Embodiment 1
[0040] (1) Carbonylation reaction
[0041] In an autoclave with a volume of 1 L, add 400 mL of absolute ethanol, 400 mmol of (S)-epichlorohydrin (ee>99.9%), 5.0 mmol of Co 2 (CO) 8 , 10 mmol of 3-hydroxypyridine. Seal the reactor, replace the reactor with carbon monoxide 3 times, and seal the reactor. In the Schlenk vacuum line, the reaction system was replaced three times with carbon monoxide gas at room temperature, and the pressure of CO gas was charged to 6.0 MPa. The temperature was controlled by a temperature controller to slowly rise to 60° C., reacted for 10 hours, cooled to room temperature, and unloaded.
[0042] The liquid of reaction gained is carried out qualitative analysis with Agilent 6890 / 5973 gas spectrometer, and Agilent 7890 gas chromatography carries out quantitative analysis: the conversion rate of (S)-epichlorohydrin is 99%, and (S)-4-chloro-3-hydroxyl The selectivity of ethyl butyrate is 95%. Distilled under reduced pressure to obtain 60.8 g of ethy...
Embodiment 2
[0046] (1) Carbonylation reaction
[0047] In an autoclave with a volume of 1 L, add 400 mL of anhydrous methanol, 400 mmol of (S)-epichlorohydrin (ee>99.9%), 5.0 mmol of Co 2 (CO) 8 , 5.0mmol of ZnBr 2 (pyridine) 2 . Seal the reactor, replace the reactor with carbon monoxide 3 times, and seal the reactor. In the Schlenk vacuum line, the reaction system was replaced three times with carbon monoxide gas at room temperature, and the pressure of CO gas was charged at 6.0 MPa. The temperature was controlled by a temperature controller to slowly rise to 60° C., reacted for 8 hours, cooled to room temperature, and unloaded.
[0048] The liquid obtained by the reaction is qualitatively analyzed with Agilent 6890 / 5973 gas chromatography spectrometer, and Agilent 7890 gas chromatography is used for quantitative analysis: the conversion rate of (S)-epichlorohydrin is 99.5%, and the conversion rate of (S)-4-chloro-3-hydroxy The selectivity of methyl butyrate was 91%, and 51.2 g of (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com