Preparation method and application of controlled-release antibiotic composite hydrogel
A composite hydrogel and antibiotic technology, which is applied in the fields of polymer chemistry and biomaterials, can solve the problems of affecting aesthetics and limited therapeutic effect of mixed anaerobic infection, and achieves high antibacterial efficiency, good tissue adhesion performance, and simple synthesis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
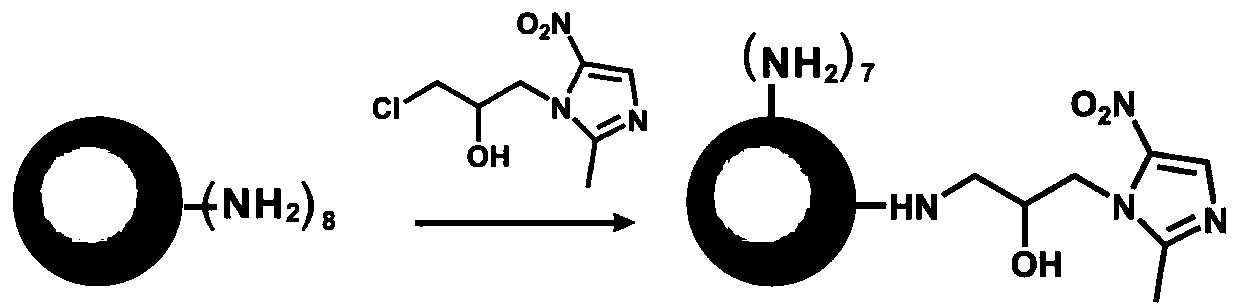
[0057] Embodiment 1: the preparation of G1-ornidazole
[0058] 250mg G1 and 153.6mg ornidazole were formulated into 100mg / mL and 50mg / mL DMSO solutions respectively, and the DMSO solution of ornidazole was added dropwise to G1, followed by the addition of 115.8mg potassium carbonate. Afterwards, it was stirred at a constant speed at 60° C. for two days, and the solvent was removed by lyophilization. Subsequently, the lyophilized product was dissolved in 2 mL of methanol, unreacted small molecule drugs were removed by ether precipitation, further filtered and vacuum-dried to obtain a brownish yellow powder product, which was a dendrimer prodrug of ornidazole. The synthetic route of G1-ornidazole is as follows figure 1 shown.
Embodiment 2
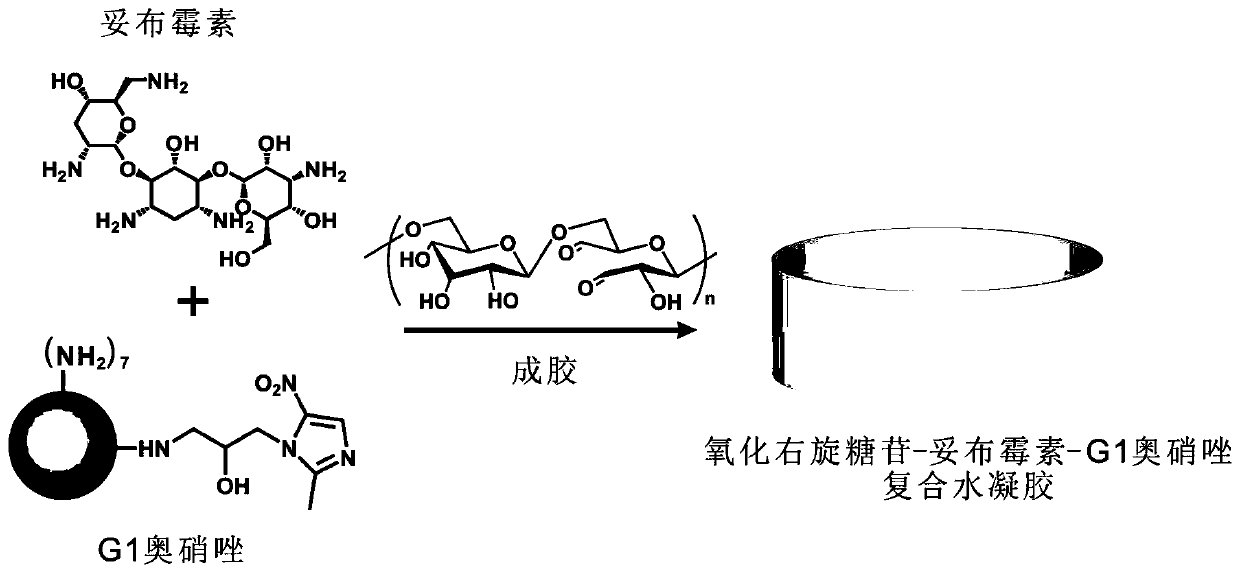
[0059] Example 2: Preparation of oxidized dextran-tobramycin-G1 ornidazole antibacterial hydrogel
[0060] The oxidized polysaccharide in this example is mainly represented by oxidized dextran as the polysaccharide polymer, and the aminoglycoside antibiotic is mainly represented by tobramycin. Mix aminoglycoside antibiotics, G1 ornidazole, and oxidized dextran. synthetic route such as figure 2 shown.
[0061] Mix 25 μL of oxidized dextran solution (30% oxidation degree, 75 mg / mL), 12.5 μL of tobramycin solution (20 mg / mL) and 12.5 μL of L1-ornidazole solution (50 mg / mL), and form at room temperature for about 5 minutes. Gel, defined in the present invention as oxidized dextran-tobramycin-G1 ornidazole composite hydrogel A.
[0062] Mix 25 μL of oxidized dextran solution (oxidation degree 30%, 50 mg / mL), 12.5 μL of tobramycin solution (50 mg / mL) and 12.5 μL of L1-ornidazole solution (100 mg / mL), and form at room temperature for about 5 minutes. Gel, defined in the present ...
Embodiment 3
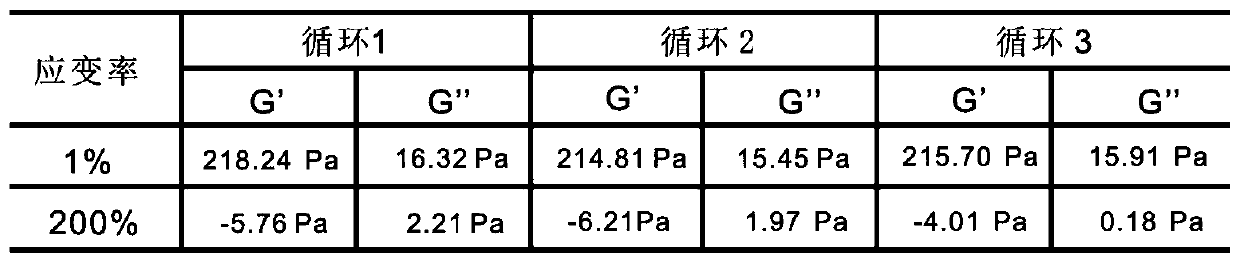
[0068] Example 3: Schematic diagram of the thixotropic properties of oxidized dextran-tobramycin-G1 ornidazole hydrogel
[0069] Preparation of oxidized dextran-tobramycin-G1 ornidazole hydrogel:
[0070] Mix 300 μL of oxidized dextran solution (oxidation degree 50%, 50 mg / mL), 50 μL of tobramycin solution (20 mg / mL) and 150 μL of LG1-orni solution (200 mg / mL), and form a gel at room temperature for about 5 minutes. Transfer the gel to the plate of the multifunctional rheometer at 37°C, set the strain rate to 1% and 200%, alternately perform time-dependent modulus measurement, and repeat 3 times to detect the thixotropy of the gel performance. Such as image 3 As shown, it can be seen that the composite gel still maintains a high storage modulus after three damage-recovery experiments, indicating that the gel has good thixotropic properties.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| storage modulus | aaaaa | aaaaa |
| modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com