Test strip for simultaneously detecting influenza A and B viruses and preparation method
A type of influenza B virus, type A technology, applied in the field of medical testing, can solve the problems of inability to guarantee the quality of the test unit, poor sensitivity and repeatability, and affect the test results, so as to facilitate clinical diagnosis, improve stability and sensitivity. , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] A preparation method for preparing the time-resolved immunochromatographic test strips for the detection of influenza A and B viruses, comprising the following steps:
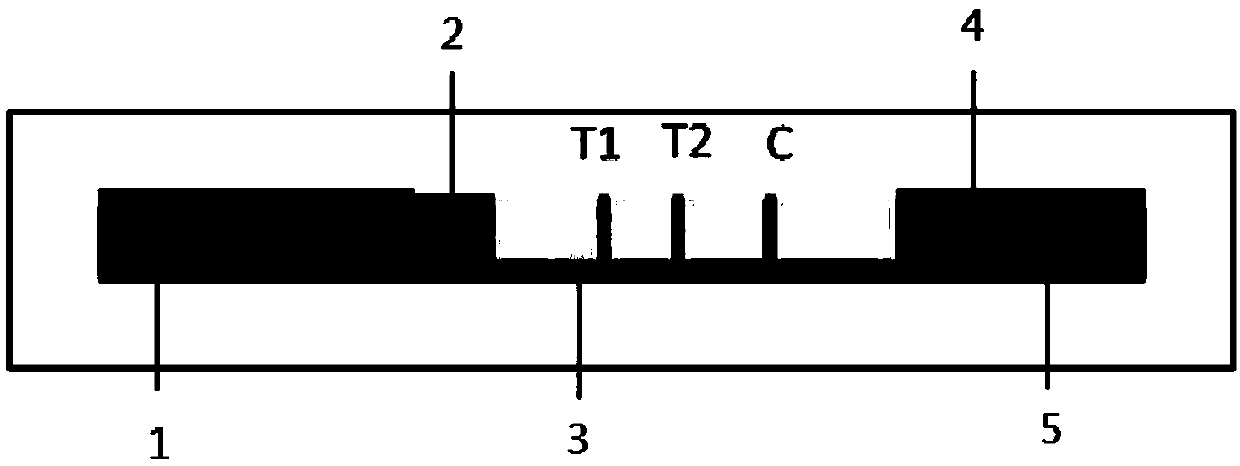
[0048] (1) respectively immobilizing the monoclonal capture antibody of type A and type B influenza virus and goat anti-mouse IgG antibody recognizing a single antigenic epitope on the reaction membrane 3, forming the first detection area, the second detection area and the control area;
[0049] (2) Prepare time-resolved fluorescent microsphere-labeled monoclonal detection antibodies for influenza A and B viruses, and use the prepared time-resolved fluorescent microsphere-labeled monoclonal detection antibodies for influenza A and B viruses in a ratio of 1:1 Mix and spray on bonding pad 2;
[0050] (3) Paste the sample pad 1, the binding pad 2, the reaction membrane 3 and the water absorption pad 4 on the bottom plate 5 in sequence to obtain the time-resolved fluorescence immunochromatography test strip....
Embodiment 1
[0075] Example 1 Time-resolved immunochromatographic test strip for simultaneous detection of influenza A and B viruses.
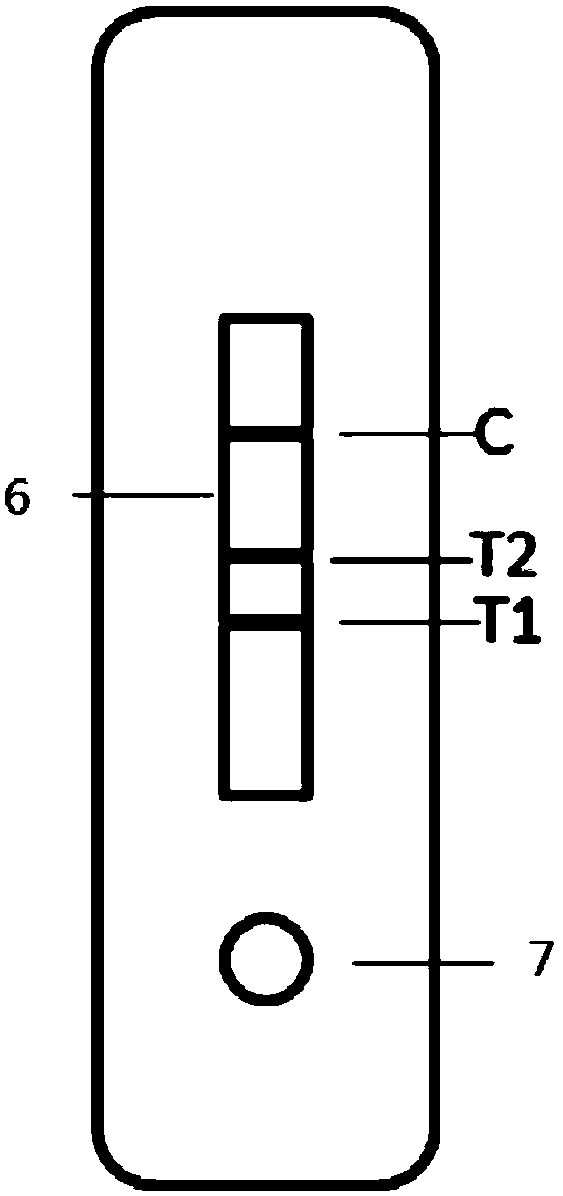
[0076] A time-resolved immunochromatographic test strip for detecting influenza A and B viruses in this embodiment includes a bottom plate, and a sample pad, a binding pad, a reaction membrane and a water absorption pad sequentially arranged on the bottom plate. The binding pad is coated with an equivalent number of fluorescent microsphere-labeled influenza A and B influenza virus monoclonal detection antibodies, and the reaction membrane includes a first detection zone T1 and a second detection zone T2 that are arranged in parallel and spaced 2mm and 4mm apart from each other. and the control area, the first detection area T1 and the second detection area T2 are close to the binding pad, the control area is close to the water-absorbing pad, and the first detection area T1 and the second detection area T2 are respectively coated with Influenza A and B viru...
Embodiment 2
[0086] Example 2 Time-resolved immunochromatography kit for simultaneous detection of influenza A and B viruses.
[0087] The time-resolved fluorescence immunochromatography kit for simultaneous detection of influenza A and B viruses in this embodiment includes: the test strip described in Embodiment 1, a plastic cartridge, and a sample buffer.
[0088] In this example, the sample buffer contains 0.5% BSA, 0.05% Tween-20 in PBS pH7.4 buffer solution.
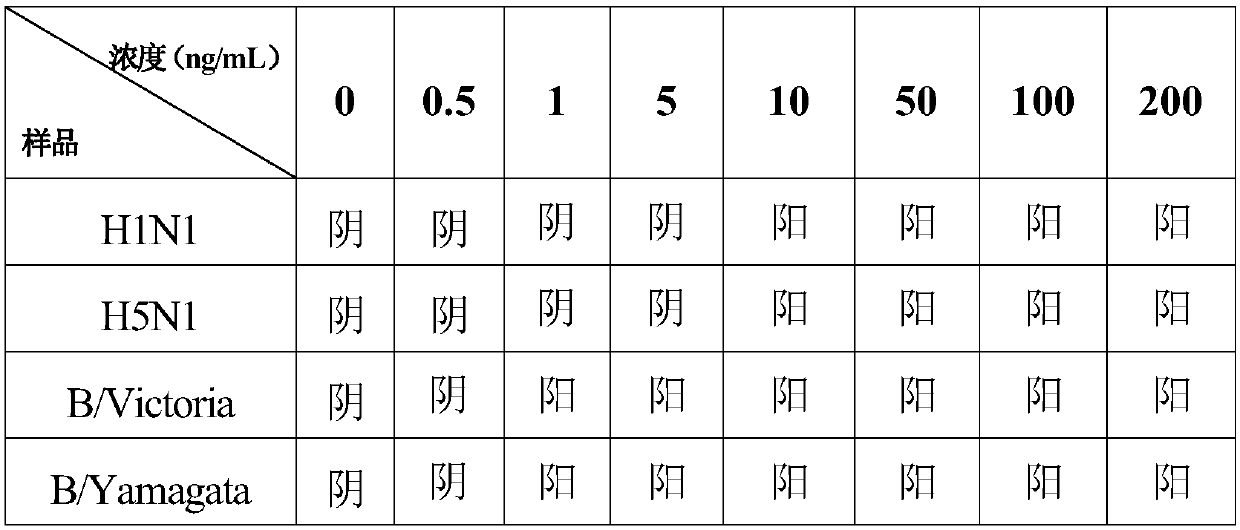
[0089] When using the time-resolved fluorescence immunochromatography kit for simultaneous detection of influenza A and B viruses of the present invention, the throat swab sample to be tested is added to the sample buffer, 50 μL of sample diluent is dropped on the sample pad of Capillary action transports the sample to the binding pad. When the throat swab sample contains influenza A and B viruses, it forms an antigen-antibody complex with the monoclonal antibody on the fluorescent microspheres. With the chromatographic action,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com