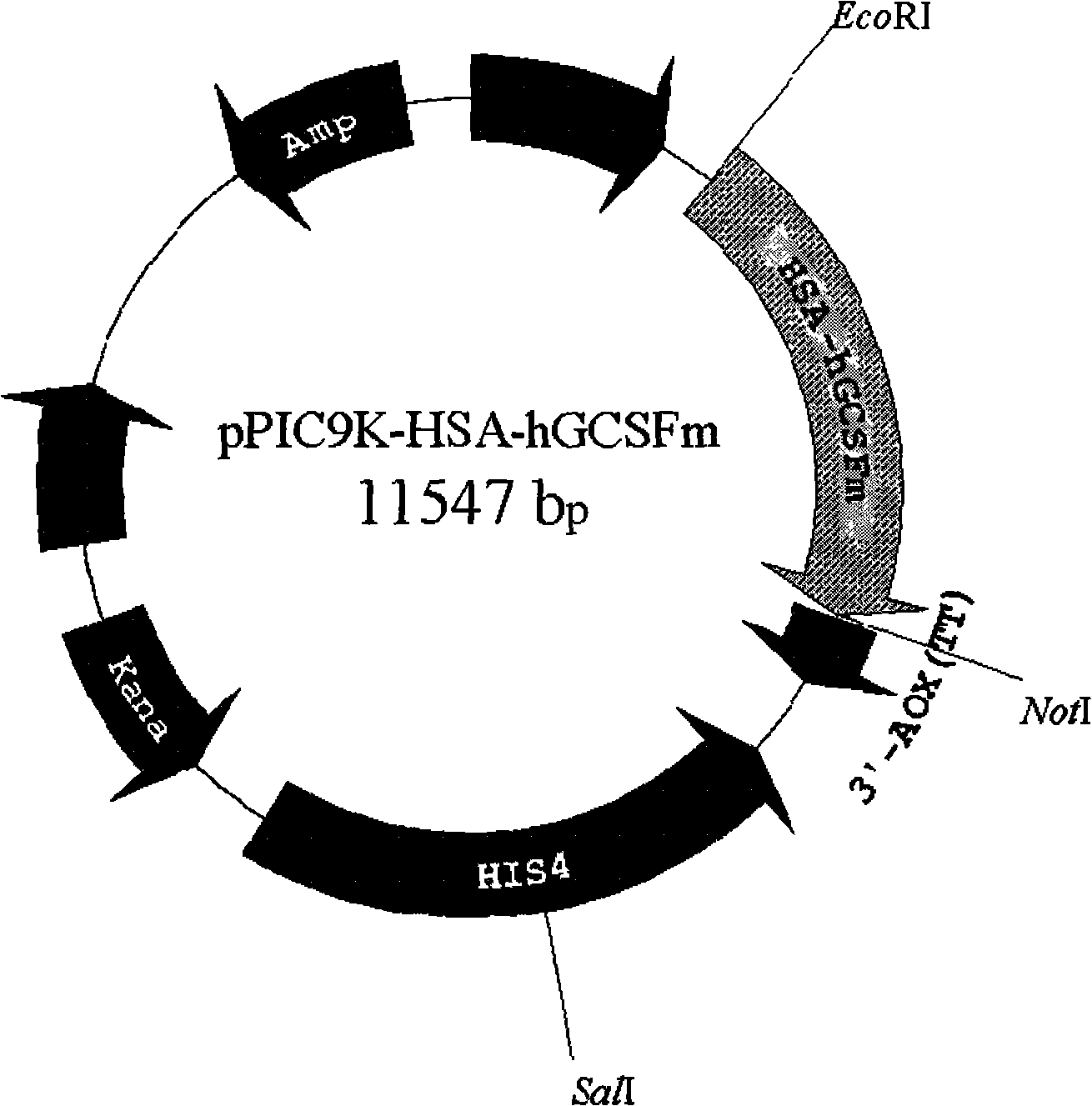
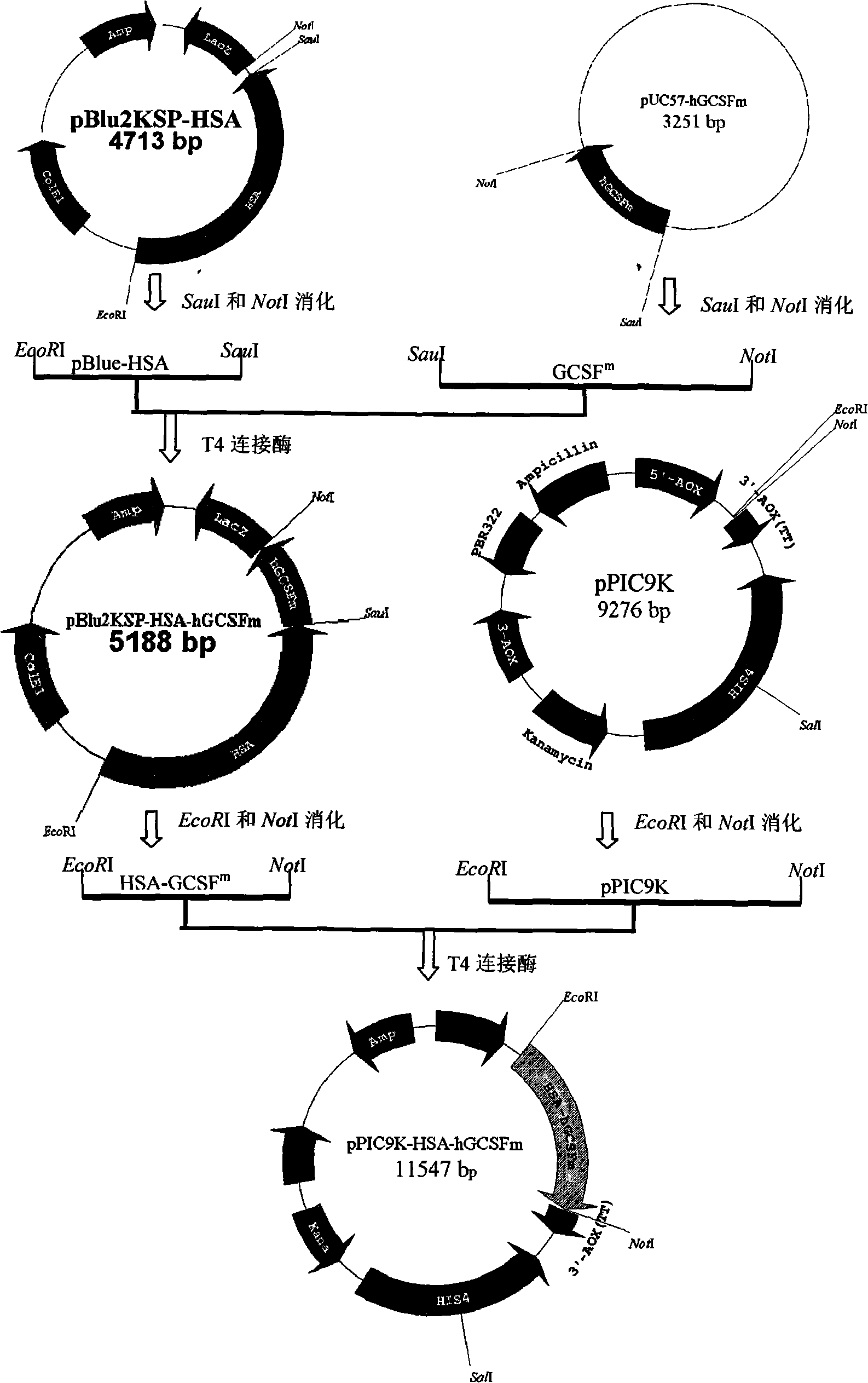
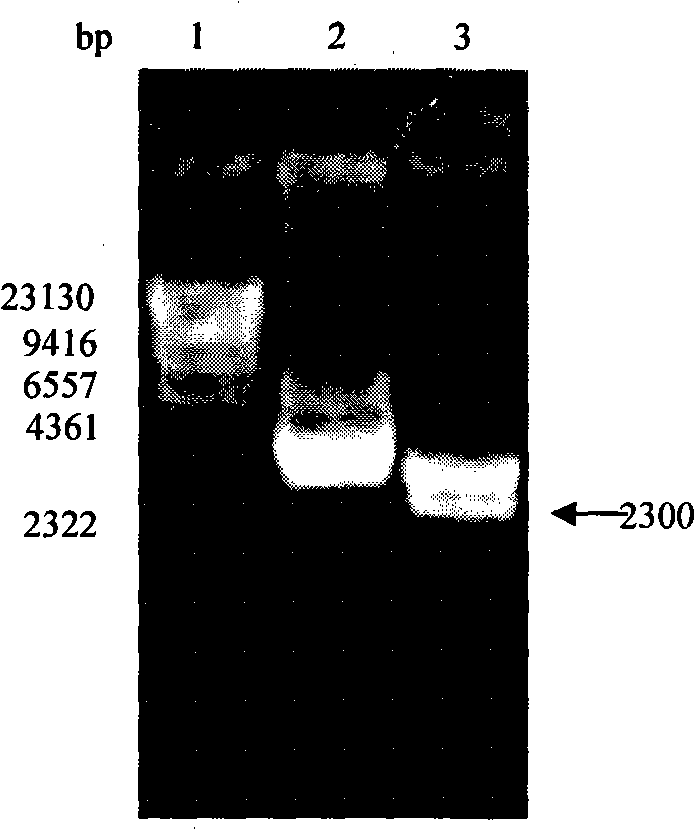
Fused protein of human serum albumin and human granulocyte colony stimulating factor mutant, and preparation thereof
A technology of human serum albumin and colony-stimulating factor, which can be applied in the directions of hybrid peptides, introduction of foreign genetic material and peptides using vectors, etc., can solve the problems of increasing patient suffering, low actual dosage, and short circulation half-life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: hGCSF m cDNA cloning
[0036] hGCSF was artificially synthesized by Shanghai Sangon Biotechnology Service Co., Ltd. m The cDNA (541bp) was cloned into the vector pUC57, the insertion site was EcoR V, and the recipient bacterium was Escherichia coli DH5α strain.
Embodiment 2
[0037] Example 2: Cloning of HSA cDNA
[0038] HSA cDNA was amplified from the human fetal liver cDNA library by PCR, and the primers used were:
[0039] PH1: 5'-CAGC GAATTC GATGCACACAAGAGTGAGGTTGCTC-3'
[0040] PH2: 5'-CACC GCGGCCGCT TTATAAGCCTAAGGCAGCTTGACTT-3'
[0041] The underlined part of PH1 is the EcoR I restriction site, and the underlined part of PH2 is the NotI restriction site
[0042] PCR reaction system: 1.5 μL of 10 μmol / L PH1 and PH2 primers, 4 μL of 2.5 mmol / L dNTP, 5 μL of 10×pfu Buffer, 0.5 μL of 5 U / μL pfu DNA polymerase, 1 μg of human fetal liver cDNA library, plus double Make up 50 μL with distilled water. PCR reaction program: pre-denaturation at 95°C for 5 min; denaturation at 94°C for 1 min, annealing at 60°C for 1 min, extension at 72°C for 3 min, 30 cycles; extension at 72°C for 10 min.
[0043] The reaction product was analyzed by agarose gel electrophoresis, and the target band appeared in the loading lane, and the 1.8kb target fragment was...
Embodiment 3
[0044] Example 3: Containing HSA cDNA and hGCSF m Construction of Cloning Vector of cDNA Fusion Gene
[0045] (1) The constructed pBlu2KSP-HSA vector contains Saul I and Not I restriction sites at the C-terminus of the HSA gene, which can be used in hGCSF m The upstream and downstream primers of the gene were respectively introduced with Saul I and Not I restriction sites, designed to make hGCSF m After the gene was digested by Saul I and Not I, it was ligated in frame with pBlu2KSP-HSA which was digested by Saul I and Not I.
[0046] (2) In-frame digestion and ligation of HSA cDNA and hGCSF m cDNA fusion gene
[0047] Digest pUC57-hGCSF with Saul I and Not I m and pBlu2KSP-HSA vector, use the PCR Fragment Gel Recovery Kit to recover hGCSF m gene and pBlu2KSP-HSA vector. The two recovered fragments were ligated with T4 DNA ligase and transformed into Escherichia coli DH5α. The transformant was spread on an LB agar plate containing X-gal, IPTG and 100 μg / mL ampicillin, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com