Preparation method and pharmaceutical composition of PEGylated recombinant human granulocyte colony-stimulating factor
A technology of PEGylation and stimulatory factors, which is applied in the field of preparation of recombinant human granulocyte stimulatory factors, can solve the problems of unfavorable industrial production, complicated operation steps, increased costs, etc., and achieve fewer types of reagents, simple process, and improved safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Take 1 L of mother solution of recombinant human granulocyte-stimulating factor (containing 1 grhG-CSF, 20 mmol NaAc and 50 mmol NaCl), cool down to 4 °C, and add 0.5 mol / L NaOH solution to adjust the pH to 6. Add methoxy-polyethylene glycol-propionaldehyde (mPEG-PAL, molecular weight about 20 kilodaltons), stir to dissolve, add sodium nitrile borohydride (NaBH3CN), and make the final concentration of sodium nitrile borohydride 15mmol / L, stirring at low speed for 15 hours, adding 0.5 mol / L hydrochloric acid solution to adjust the pH of the reaction solution to 3.
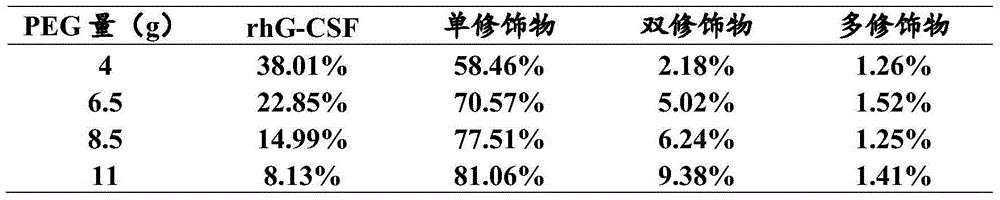
[0041] According to the different amounts of mPEG-PAL added, the ratio of rhG-CSF monomer, PEG single modification of rhG-CSF (PEG-rhG-CSF), PEG double modification of rhG-CSF, and PEG multi-modification of rhG-CSF in the reaction solution The ratio is shown in Table 1.
[0042] The reaction solution was purified by a chromatographic column, and the filler was SPSepharoseFF, which was first equilibrated in ...
Embodiment 2
[0046] Take 1 L of mother solution of recombinant human granulocyte-stimulating factor (containing 1 grhG-CSF, 20 mmol NaAc and 50 mmol NaCl), lower the temperature to 10° C., and add 1 mol / L NaOH solution to adjust the pH to 5.5. Add 7 g of methoxy-polyethylene glycol-propionaldehyde (mPEG-PAL, molecular weight about 20 kilodaltons), stir to dissolve, add sodium nitrile borohydride (NaBH3CN), stir at low speed for 18 hours, add 0.5 mol / L Hydrochloric acid solution adjusted the pH of the reaction solution to 4.
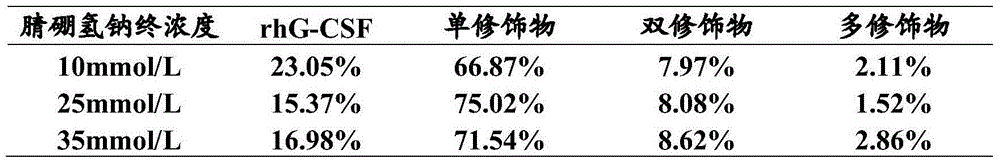
[0047] According to the final concentration of sodium nitrile borohydride, the rhG-CSF monomer, the PEG single modification of rhG-CSF (PEG-rhG-CSF), the PEG double modification of rhG-CSF, and the PEG multi-modification of rhG-CSF in the reaction liquid were different. The proportions of the substances are shown in Table 2.
[0048] The reaction solution was purified by a chromatographic column, and the filler was SPSepharoseFF, which was first equilibrated in the e...
Embodiment 3
[0052] Take 1 L of mother solution of recombinant human granulocyte-stimulating factor (containing 1 grhG-CSF, 20 mmol NaAc and 50 mmol NaCl), lower the temperature to 8°C, and add 1 mol / L NaOH solution to adjust the pH to 5-7. Add 6 g of methoxy-polyethylene glycol-propionaldehyde (mPEG-PAL, molecular weight about 20 kilodaltons), stir to dissolve, add sodium nitrile borohydride (NaBH CN), and make the final concentration of sodium nitrile borohydride 35 mmol / L, stirred at low speed for 16 hours, and added 1mol / L hydrochloric acid solution to adjust the pH of the reaction solution to 3.5.
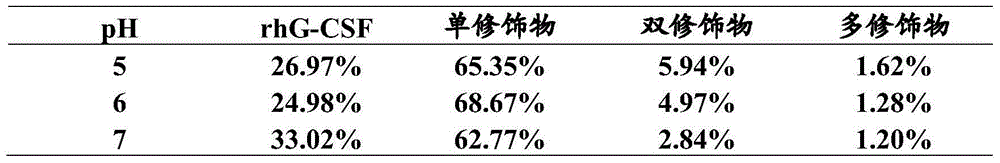
[0053] According to the pH adjusted by the sodium hydroxide solution, the rhG-CSF monomer, the PEG single modification of rhG-CSF (PEG-rhG-CSF), the PEG double modification of rhG-CSF, and the PEG multi-modification of rhG-CSF in the reaction liquid The proportions of the substances are shown in Table 3.
[0054] The reaction solution was purified by a chromatographic column, and the fil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com