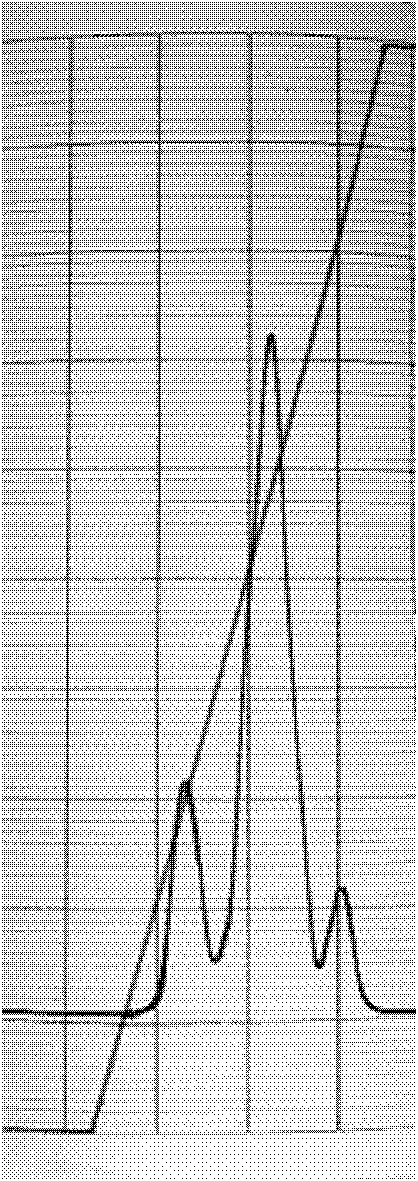Purification method of pegylated recombinant human granulocyte colony stimulating factor
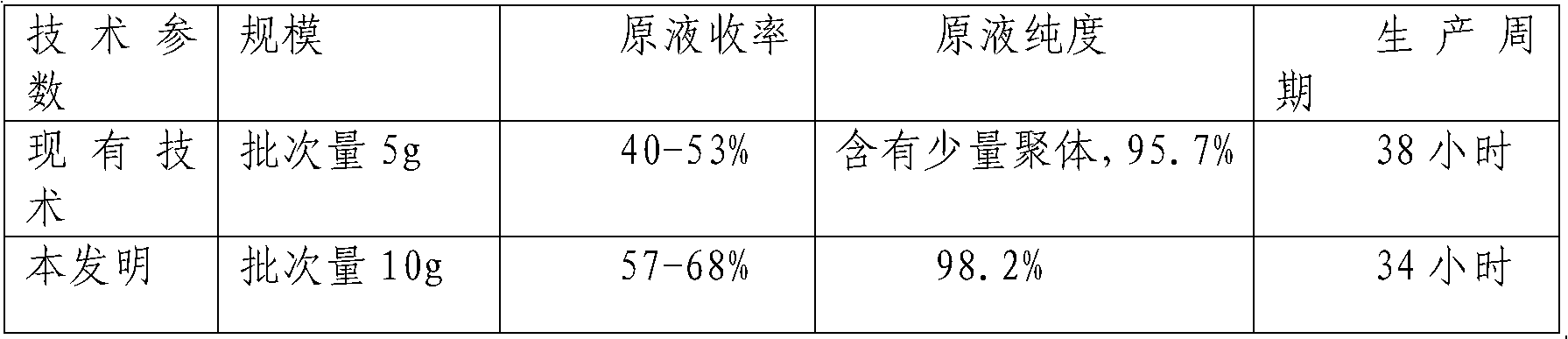
A technology of colony stimulating factor and pegylation, applied in the field of medicine, can solve the problems of difficult control of endotoxin content, difficult amplification of molecular sieves, difficult control of endotoxin, etc., and achieves less loss of target protein and improved protein yield and quality. , easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Equilibrate the Sephadex G-25 desalting chromatography column with 100 mM phosphate buffer solution at pH 8.5, load the PEG-G-CSF cross-linking solution, and collect the desalting solution.
[0032] 2. First connect the anion exchange chromatography column and the multi-binding ion exchange chromatography column in series. The chromatography medium of the anion exchange chromatography column is agarose gel (sepharose Q Fast Flow), and the multi-binding ion exchange chromatography column The chromatographic medium used was high flow rate agarose gel (Captro adhere).
[0033] 3. Equilibrate the tandem chromatography column with pH 8.5 100mM phosphate buffer, load the collected desalted solution, and then wash with pH 8.5 100mM phosphate buffer to the baseline. Then separate the anion-exchange chromatography column from the multi-binding ion-exchange chromatography column, equilibrate the Captro adhere ion-exchange chromatography column with 100mM Tris-HCl buffer soluti...
Embodiment 2
[0037] 1. Equilibrate the Sephadex G-25 desalting chromatography column with 10 mM phosphate buffer solution at pH 6.0, load the PEG-G-CSF cross-linking solution, and collect the desalting solution.
[0038] 2. First connect the anion exchange chromatography column and the multi-binding ion exchange chromatography column in series. The chromatography medium of the anion exchange chromatography column is agarose gel (sepharose Q Fast Flow), and the multi-binding ion exchange chromatography column The chromatography medium used was high-flow agarose gel (Captro MMC).
[0039] 3. Equilibrate the tandem chromatography column with 10mM phosphate buffer solution of pH 6.0, load the collected desalted solution, and then wash to the baseline with 10mM phosphate buffer solution of pH 6.0. Then separate the anion exchange chromatography column from the multi-binding ion exchange chromatography column, equilibrate the Captro MMC ion exchange chromatography column with 100mM sodium acetat...
Embodiment 3
[0043] 1. Equilibrate the Sephadex G-25 desalting chromatography column with 20 mM phosphate buffer solution at pH 8.0, load the PEG-G-CSF cross-linking solution, and collect the desalting solution.
[0044] 2. First connect the anion exchange chromatography column and the multi-binding ion exchange chromatography column in series. The chromatography medium of the anion exchange chromatography column is agarose gel (sepharose DEAE Fast Flow), and the multi-binding ion exchange chromatography column The chromatographic medium used was high flow rate agarose gel (Captro adhere).
[0045] 3. Equilibrate the tandem chromatographic column with 20mM phosphate buffer of pH 8.0, load the collected desalted solution, and then wash to the baseline with 20mM phosphate buffer of pH 8.0. Then separate the anion-exchange chromatography column from the multi-binding ion-exchange chromatography column, equilibrate the Captro adhere ion-exchange chromatography column with 20mM Tris-HCl buffer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com