Method for producing recombinant human granulocyte colony stimulating factor
A technology of colony-stimulating factor and production method, which is applied in the biological field and can solve the problems of low renaturation rate and high production cost of recombinant human granulocyte colony-stimulating factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Fermentation: Inoculate the engineered strains on the LA plate, cultivate them at 30°C, and after they grow into colonies, pick a single colony and inoculate it in 10ml LA liquid culture medium, shake it at 30°C to obtain a first-grade seed liquid, and transfer it to 1% in LA liquid medium, shaking culture to obtain secondary seed liquid.
[0028] Prepare the fermentation medium, put the part that can withstand high pressure into the fermenter for high-pressure sterilization, filter and sterilize the components that cannot withstand high pressure, add it after the liquid in the fermenter cools down, and add the newly cultivated seed liquid at 10% Proportional inoculation in fermenter for fermentation. Control the culture temperature at 30°C, adjust the pH value with ammonia water to stabilize the pH between 6.8 and 7.4, control the dissolved oxygen > 70% by adjusting the ventilation volume and rotation speed, and feed appropriately. Enrichment to OD 600 At 2.5-3.0, th...
Embodiment 2
[0030] Bacteria destruction and inclusion body washing: suspend the wet bacteria with TE (50mmol / LTris-Cl, 5mmol / LEDTA) at a ratio of 1:10 (g / ml), add lysozyme to a final concentration of 1mg / ml, 4 Place at ℃ for 4 hours, and ultrasonically break in an ice bath, 6 minutes each time, with an interval of 3 minutes, a total of 7 times. Centrifuge at 4°C and 8500 rpm for 15 min, discard the supernatant, resuspend the pellet in TE buffer, repeat the above process twice, and wash the pellet twice with 2 mol / L urea.
[0031] The precipitate was dissolved with 8mmol / L urea, 2mmol / L EDTA, 1mmol / L DTT, 50mmol / L Tris-HCl pH 8.0, centrifuged at 8500rpm at 4°C for 15min, and the supernatant was the inclusion body eluate.
Embodiment 3
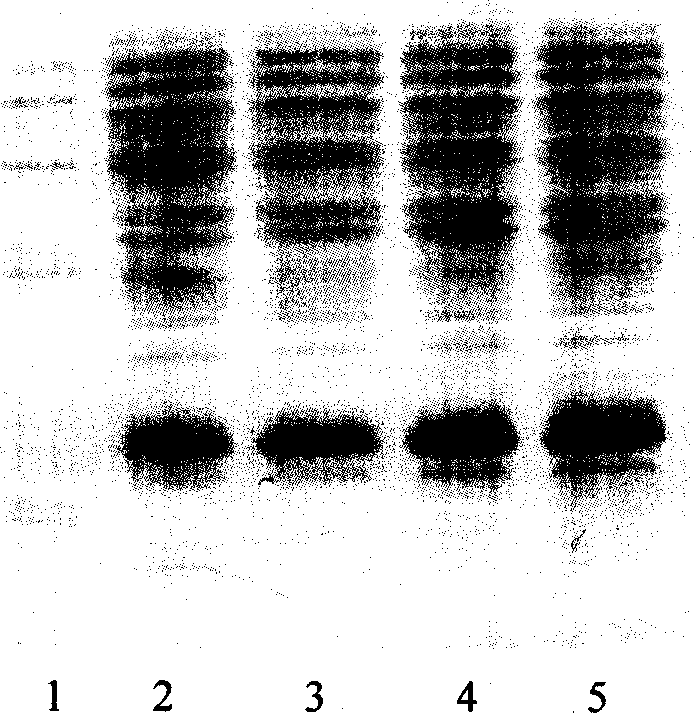
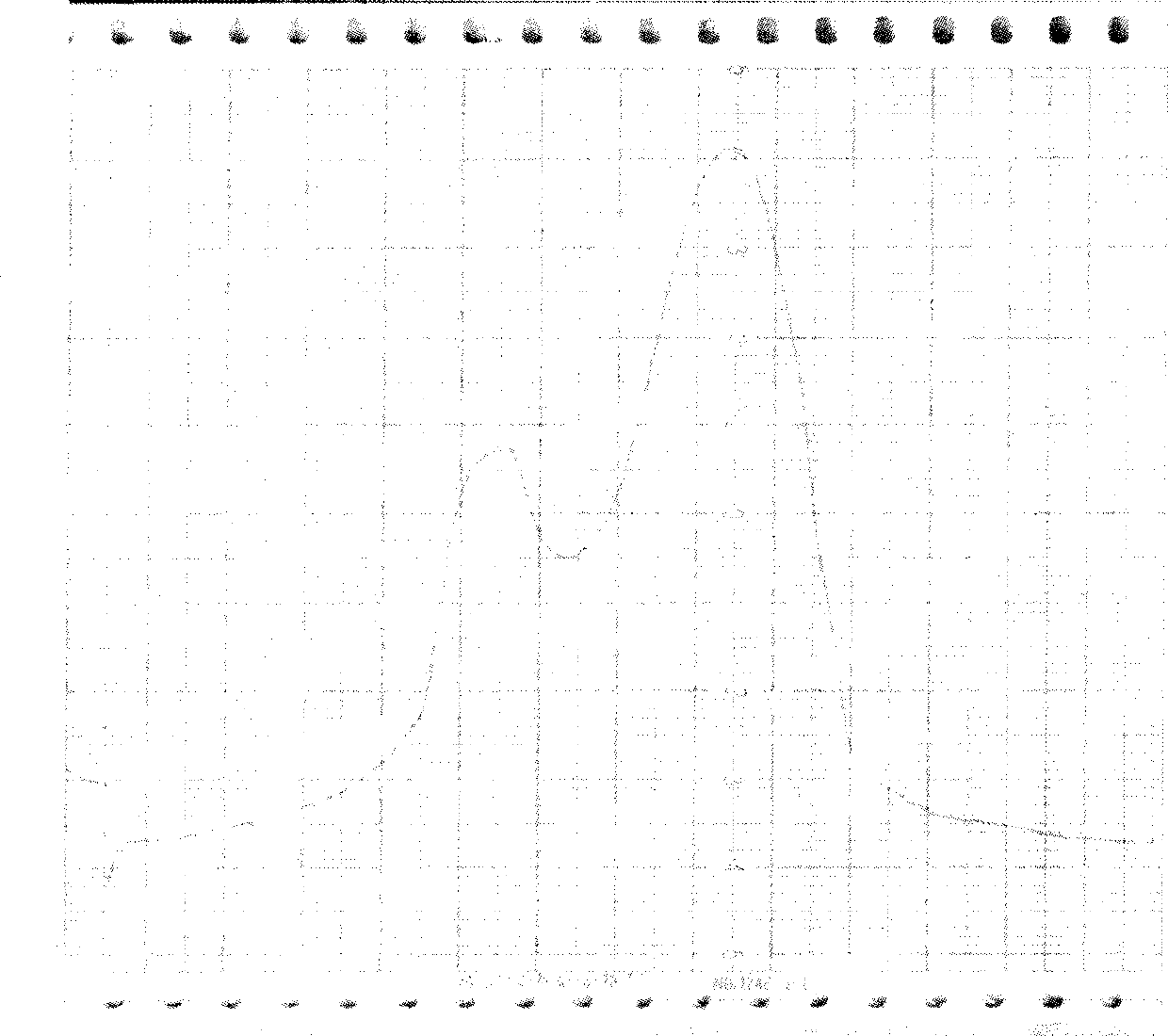
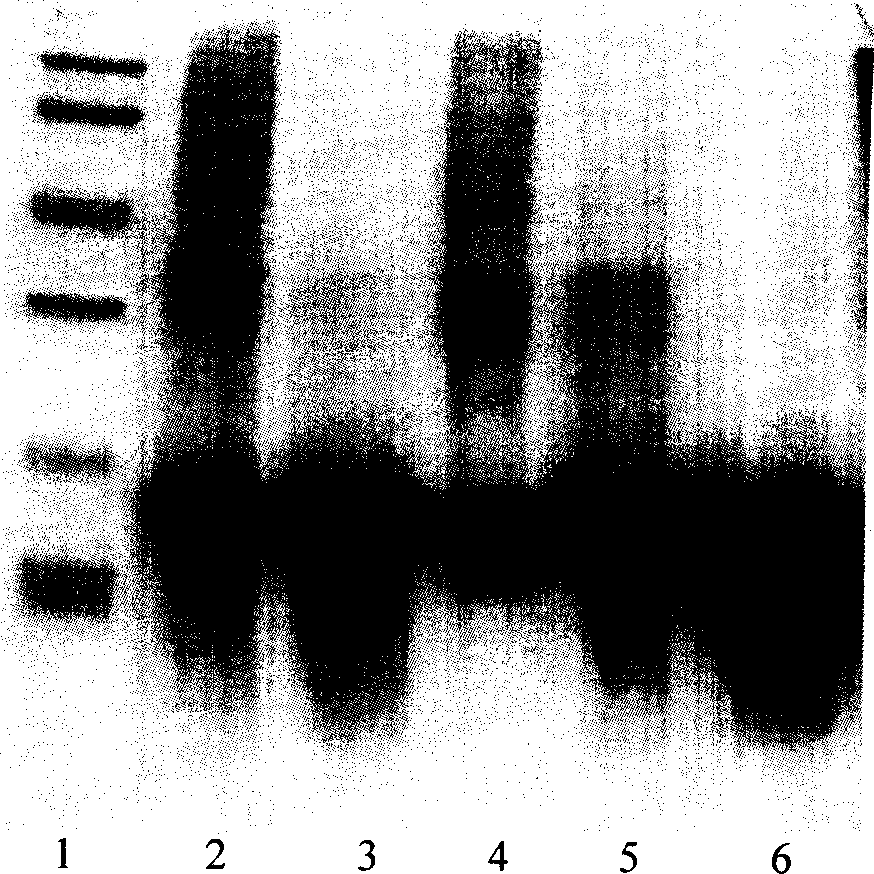
[0033] Molecular sieve chromatography: take the above-mentioned inclusion body dissolution solution, add DTT to a final concentration of 5mmol / L, put it on the column in a water bath at 30°C for 30min, and the mobile phase is 8mmol / L urea, 2mmol / L EDTA, 1mmol / L LDTT, 50mmol / L Tris-HCl pH 8.0, the flow rate is about 0.7ml / min, and the elution peaks are collected in sections. The chromatographic curve is shown in Figure 2. The target protein is located in the second peak, and the impurity protein with a molecular weight greater than 30KD can be removed by molecular sieve purification, as shown in Figure 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com